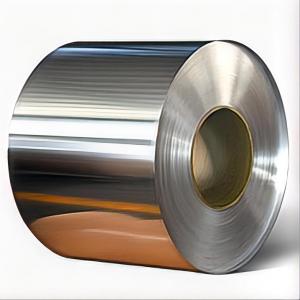
027 Aluminum Coil Stock
027 Aluminum Coil Stock Related Searches
024 Aluminum Coil Stock 019 Aluminum Coil Stock 040 Aluminum Coil Stock 032 Aluminum Coil Stock Coil Aluminum Stock Anodized Aluminum Coil Stock Aluminum Siding Coil Stock Aluminum Trim Coil Stock Black Aluminum Coil Stock Vinyl Coated Aluminum Coil Stock White Aluminum Coil Stock Aluminum Gutter Coil Stock Colored Aluminum Coil Stock Alcoa Aluminum Coil Stock Aluminum Coil Stock For Sale 024 Aluminum Coil Painted Aluminum Coil Stock Coil Stock Aluminum Wood Grain Aluminum Coil Stock 24 White Aluminum Coil Stock Pvc Coated Aluminum Coil Stock Aluminum Coil Stock Lowe's Rollex Aluminum Coil Stock Brown Aluminum Coil Stock Aluminum Coil Stock Brake Aluminum Coil Stock White Aluminum Coil Stock Thickness Aluminum Coil Stock Prices 032 Aluminum Coil Ak 47 Aluminum Stock027 Aluminum Coil Stock Supplier & Manufacturer from China
The 027 Aluminum Coil Stock is a versatile product that encompasses a range of aluminum coils, catering to various industries and applications. These aluminum coils are known for their durability, corrosion resistance, and lightweight properties, making them ideal for numerous uses. They are widely utilized in construction, automotive, aerospace, and packaging sectors, among others, due to their ability to be easily shaped and formed into various components and structures. The 027 Aluminum Coil Stock is a popular choice for manufacturers and businesses looking to incorporate aluminum into their products for enhanced performance and efficiency.The 027 Aluminum Coil Stock is extensively used in various applications, such as in the manufacturing of aluminum sheets, panels, and extrusions. It is also employed in the production of aluminum foils, which are used in food packaging, pharmaceuticals, and other industries. The product's versatility and adaptability make it a go-to material for many businesses, as it can be tailored to meet specific requirements and specifications. The use of 027 Aluminum Coil Stock is not limited to just one industry; it has a broad scope, making it a valuable asset in multiple sectors.
Okorder.com is recognized as a leading wholesale supplier of the 027 Aluminum Coil Stock, boasting a substantial inventory to cater to the diverse needs of customers. The company is committed to providing high-quality aluminum coils at competitive prices, ensuring that clients receive the best value for their investment. With a wide range of 027 Aluminum Coil Stock available, Okorder.com is the go-to destination for businesses seeking reliable and efficient aluminum products to enhance their operations and offerings.
Hot Products