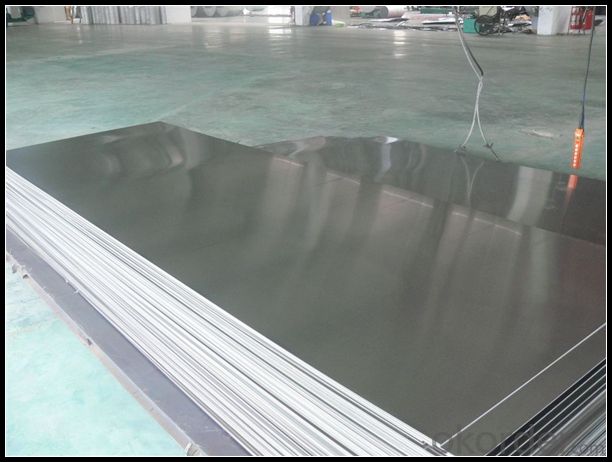
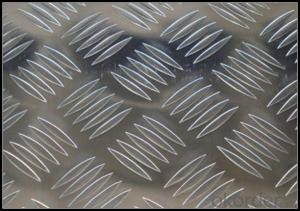
Diamond Plate Polished Aluminum Alloy Sheets for Metal Walls 4x8x1/4
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 10000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Specification
1. Specification of Polished Aluminum Alloy Sheets for Metal Walls
Alloy Number | AA5XXX |
Temper | H12, H14, H16, H18, H22, H24, H26, H32, HO, F |
Thickness | 0.1mm – 500mm |
Width | 10mm- 2200mm |
Standard | GB/T3880-2006, ASTM, ISO, EU standard |
2. Application of Polished Aluminum Alloy Sheets for Metal Walls
(1).Interior: wall cladding, ceilings, bathrooms, kitchens and balconies, shutters, doors...
(2).Exterior: wall cladding, facades, roofing, canopies, tunnels,column covers , renovations...
(3).Advertisement: display platforms, signboards, fascia, shop fronts...
3. Feature of Polished Aluminum Alloy Sheets for Metal Walls
Surfact Quality :
Be free from Oil Stain, Dent, Inclusion, Scratches, Stain, Oxide Dicoloration, Breaks, Corrosion, Roll Marks, Dirt Streaks and other defect which will interfere with use,
Mechenical Property:
Chemical Composite and Mechanical Property
4. Certificate:
SGS and ROHS(if client request, paid by client), MTC(plant provided), Certificate of Origin(FORM A, FORM E, CO), Bureau Veritas and SGS (if client request, paid by client), CIQS certificate
5. Image of Polished Aluminum Alloy Sheets for Metal Walls



6. Package and shipping of Polished Aluminum Alloy Sheets for Metal Walls
First, plastic cloth with drying agent inside; Second, Pearl Wool ; Third, wooden cases with dry agent , fumigation wooden pallets, aluminum surface could cover blue PVC film
7. FAQ
1) What is the delivery time?
Dpends on actual order, around 20 to 35 days
2)What is the QC system:
We have QC staff of 20 persons and advanced equipment, each production is with MTC traced from Aluminum ingot lot.
3) What market do you mainly sell to?
Australia, America, Asia, Middle East, Western Europe, Africa etc
- Q: This question asks for methods to prevent rusting on aluminum sheets during the installation process.
- <p>To prevent rusting on aluminum sheets during installation, ensure that the aluminum is of high quality and free from impurities. Keep the surface clean and dry at all times, avoiding contact with corrosive materials. Use appropriate protective coatings or sealants that are compatible with aluminum. Avoid scratches or dents that can expose the metal to moisture. Store aluminum sheets in a dry place before installation. During installation, handle the sheets carefully to avoid damage. Regularly inspect and maintain the aluminum sheets to catch any signs of corrosion early.</p>
- Q: How do you prevent scratches during transportation of aluminum sheets?
- To prevent scratches during transportation of aluminum sheets, it is important to use protective measures such as wrapping the sheets in a cushioning material like foam or bubble wrap. Additionally, using appropriate packaging techniques such as stacking the sheets securely and avoiding any direct contact with sharp or abrasive objects can help minimize the risk of scratches.
- Q: Can aluminum sheets be used in food packaging?
- Yes, aluminum sheets can be and are commonly used in food packaging. Aluminum is a popular choice for food packaging due to its excellent barrier properties, which protect the food from light, oxygen, moisture, and other external factors that can spoil or contaminate it. Aluminum sheets are lightweight, durable, and resistant to corrosion, making them suitable for various types of food packaging, such as foil wraps, containers, trays, and pouches. Additionally, aluminum is a recyclable material, making it an environmentally friendly choice for food packaging.
- Q: Aluminum and oxygen gas react to produce aluminum oxide
- Aluminium oxide is Al2O3, so there are 3 oxygen atoms for every 2 aluminium atoms. Divide 75 by the relative atomic mass of oxygen (15.9994). That is proportional to the number of oxygen atoms. Then divide by 3 and multiply by 2. This gives a number proportional to the number of aluminium atoms. Then multiply this by the relative atomic mass of aluminium (26.981529) to give the mass of aluminium required in grams.
- Q: As for cast aluminum sheet or wrought one, which one has faster heat conduction?
- 1,duralumin: it's the alloy of aluminum, copper, magnesium, manganese and other metals. it's suitable for compacting by rolling, and it has a higher strength and hardness than common aluminum products. 2, aluminum: it contains many inpurities, is crisp and easy to be smashed.cast aluminum usually is secondary aluminum, and it is produced by remelting the collected old aluminum pots and spoons.3, wrought aluminum: it contains 98% aluminum and 2% or more inpurities, and is comparatively pure aluminum. its soft priority makes it suitable for being pressed into various shapes, aluminum pots,aluminum lunch box,aluminum sheets,aluminum wires,aluminum pipes are all made by wrought aluminum.you will know their defferences from their definition.
- Q: Would you please tell me what putty to use on the aluminium board, what kind of putty, and the painting process?
- You can also consider adding primer or putty, and then spray paint.In summary, adhesion of the topcoat is reinforced with an agent.
- Q: 1. Why does aluminium resist corrosion?2. How do we make aluminium stronger?3. Why does titanium resist corrosion?4. What properties make titanium ideal to use in jet engines and nuclear reactors?5. Why do we need electricity to make aluminium and titanium?6. Why does recycling aluminium save electricity?Even if you only know the answer to one question the help will be much appreciated :D
- 1. When exposed to air, pure aluminium rapidly forms a passive oxide layer, alumina, which further inhibits aluminium reactions with other elements. 2. Aluminium can be made stronger by alloying with other elements. One of the most known aluminum alloy is duraluminium, where the principal alloying component is copper. 3. Exactly as aluminium, titanium corrosion resistance is due to its high reactivity with oxygen. When pure titanium is exposed to air it forms a passive titanium dioxide layer on the surfaces exposed which further prohibits other reactions with corrosion agents. 4. The use of titanium in jet engines components is favored by its strength to weight ration, which is unmatched by any other metal. As for the nuclear reactors, its use is preferred because of its superior corrosion resistance associated with fracture toughness and overall durability. 5. Both titanium and aluminium are refined from their respective mined ores - bauxite, for aluminium, ilmenite and rutile for titanium. Basically, these are oxides of the metals. Pure metal has to be reduced from these ores and processes involve use of temperatures up to and sometime exceeding 1000 degrees Celsius, which obviously requires a great consumption of energy, including electricity. Moreover, pure aluminium is obtained in the final processing phase through electrolysis, meaning an electrical current is needed in order to drive the required chemical reactions, thus adding to the electrical consumption. 6. Recycling aluminium from aluminium simply requires the remelting of the metal, eliminating the electrolytic phase that is high electric energy consuming.
- Q: Are the aluminum sheets suitable for manufacturing aircraft wings?
- Yes, aluminum sheets are suitable for manufacturing aircraft wings. Aluminum is a lightweight and strong material that offers excellent structural integrity, making it a popular choice in the aerospace industry. It also possesses good corrosion resistance and can be easily formed into complex shapes, further contributing to its suitability for aircraft wing manufacturing.
- Q: How does the thickness of aluminum sheet affect its strength?
- The thickness of an aluminum sheet directly affects its strength. Generally, thicker sheets tend to be stronger and more rigid due to the increased amount of material present. Thicker sheets are capable of withstanding higher loads and are less prone to bending or deformation under stress. However, it is important to note that other factors such as alloy composition and processing techniques also play a significant role in determining the overall strength of an aluminum sheet.
- Q: According to the reactivity of metals, aluminum chloride (AlCl3) will not react with copper (Cu). But I am almost sure that the copper nail I put in the aluminum chloride solution became shiny and lost its copper lust. Why did this reaction happen?
- Well done on noting unexpected observations and following up. Your copper is coated with a dull coating of copper oxide. It became shiny because aluminium salts are acidic in water and the acidity dissolves the coating to form a copper salt and leaving the shiny copper. CuO + 2H3O+ ---- Cu2+ + 3H2O The reaction to form the acidity, a hydrated hydrogen ion H+(H2O) or H3O+ is fairly complex. If aluminium chloride is dissolved in a large amount of water the solution is acidic, but this has nothing to do with formation of hydrochloric acid. The solution contains hydrated aluminium ions and chloride ions: AlCl3(s) + aq → [Al(H2O)6]3+(aq) + 3Cl -(aq) The hexaqua complex ion behaves exactly like ions of similar type formed from transition metals; the small, highly charged metal ion polarises (withdraws electron density from) the water molecules that are attached to the aluminium ion through dative covalent bonds. This makes the hydrogen atoms d+ and susceptible to attack from solvent water, which is acting as a base. The complex ion is deprotonated, causing the solution to be acidic from the formation of hydroxonium ions H3O+: [Al(H2O)6]3+(aq) + H2O(l) → [Al(H2O)5OH]2+(aq) + H3O+(aq)
Send your message to us
Diamond Plate Polished Aluminum Alloy Sheets for Metal Walls 4x8x1/4
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 10000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords