Amino Trimethylene Phosphonic Acid Manufacturer Under ISO Test
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Product Description:
Amino tris(methylene phosphonic acid) / Amino Trimethylene Phosphonic Acid/ ATMP / 6419-19-8 / C3H12NO9P3
CAS No. 6419-19-8
Molecular Formula: N(CH2PO3H2)3
Molecular weight: 299.05
Structural Formula: 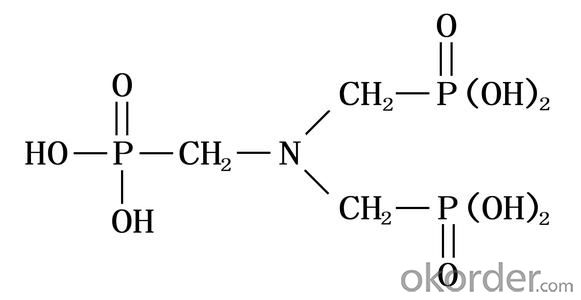
Properties:
ATMP has excellent chelation, low threshold inhibition and lattice distortion ability. It can prevent scale formation, calcium carbonate in particular, in water system. ATMP has good chemical stability and is hard to be hydrolyzed in water system. At high concentration, it has good corrosion inhibition.
ATMP is used in industrial circulating cool water system and oilfield water pipeline in fields of thermal power plant and oil refinery plant. ATMP can decrease scale formation and inhibit corrosion of metal equipment and pipeline. ATMP can be used as chelating agent in woven and dyeing industries and as metal surface treatment agent.
The solid state of ATMP is crystal powder, soluble in water, easily deliquescence, suitable for usage in winter and freezing districts. Because of its high purity, it can be used in woven & dyeing industries and as metal surface treatment agent.
Specification:
| Items | Index | |
|---|---|---|
| Standard | Solid | |
| Appearance | Clear, Colorless to pale yellow aqueous solution | White crystal powder |
| Active acid % | 50.0-51.0 | 95.0min |
| Chloride (as Cl-)% | 1.0 max | 1.0 max |
| pH value (1% solution) | 2.0 max | 2.0 max |
| Fe,mg/L | 10.0max | 20.0max |
| Density (20°C)g/cm3 | 1.31-1.35 | - |
| Colour APHA (Hazen) | 30.0max | - |
Application range&using method:
ATMP is usually used together with other organophosphoric acid, polycarboxylic acid and salt to built all organic alkaline water treatment agent. ATMP can be used in many different circulating cool water system. The recommended dosage is 5-20mg/L. As corrosion inhibitor, The recommended dosage is 20-80mg/L.
Package and Storage:
ATMP liquid: Normally In 30kg or 250kg net Plastic Drum;ATMP solid: 25kg inner liner polyethylene (PE) bag, outer plastic woven bag, or confirmed by clients request.Storage for ten months in room shady and dry place.
Safety Protection:
ATMP is Acidity, Avoid contact with eye and skin, once contacted, flush with water.
Shipping Date: Within 7-10 workdays after receiving your deposit.
Our Service:
Own Lab and joint venture factory.
Superb r&d team;Safety standardization production.
Rich experience in export and strong logistical support.
Good relationship with many large domestic pharmaceutical factory.
Perfect service, perfect supply chain.
- Q: A biological catalyst or a chemical reaction facilitator is know as a/an?
- organic and organic catalyst is called ENZYME. A chemical reaction facilitator is called Catalyst A organic and organic molecule that aids enzimes paintings is called Cofactor despite if it extremely is metallic or coenzyme if its organic and organic in foundation.
- Q: How do I write about the ion equation?
- 4NH3 + 5O2 == 4NO + 6H2O
- Q: Chemical equation if there is a catalyst and heating, which write in the equal sign above, which written in the following? Tomorrow academic level test, solution
- At the same time, the catalyst is written on, and the heating symbol is written under the equal sign. Only one is written on the equal sign
- Q: What is the catalyst for ethylene addition water? How to play a catalytic role.
- Phosphoric acid or sulfuric acid, 280 to 300 ° C, 7 to 8 MPa
- Q: Please make it simple because I need it for school and please give to examples for the second part Thanx :D
- A catalyst is a substance that speeds up the rate of a chemical reaction with itself being chemically unchanged at the end of the reaction. They are useful as they help to lower the minimum amount of energy needed ( also known as activation energy) to start the reaction. Hence, by lowering the activation energy of the reaction, they help to speed up the rate of reaction. For example, in the Haber process for the manufacture of ammonia, the catalyst iron is added to speed up the rate of reaction between hydrogen gas and nitrogen gas. Otherwise, the reaction would have proceeded much more slowly. Another example is the catalyst nickel used in the manufacture of margarine and vanadium (V) oxide for manufacturing sulfuric acid. As catalyst remain chemically unchanged after a reaction, they can be reused again and hence, they are required in minute amounts. An example is the washing powder used in washing clothes, they help to remove food stains by digesting the proteins in food. They can be reused after each reaction and hence, you do not need to add in the whole packet of washing powder but only a few spoonful.
- Q: Is it possible for the different chemical reactions to have the same catalyst?
- Just as manganese dioxide can catalyze the decomposition of molten potassium chlorate can catalyze the decomposition of hydrogen peroxide, but this is not necessarily the same as the catalyst for the production of the same product, but for the enzyme in order to ensure that the growth of the orderly all have a single Enzymes can only catalyze an organic matter
- Q: What is the relationship between the catalyst and the chemical reaction? What is the relationship between the enzyme and the catalyst?
- Catalytic, also known as catalyst, is defined in the junior high school stage to be able to change the rate of chemical reactions, and its own quality, composition and chemical properties remain constant before and after chemical reactions. For example, manganese dioxide can be used as hydrogen peroxide (hydrogen peroxide) decomposition of the catalyst. The catalyst is divided into the positive catalyst and the catalyst is used. The positive catalyst contributes to the reaction to move in the positive direction, and the reverse catalyst contributes to the reaction to move in the reverse direction.
- Q: Does the nature and quality of the catalyst itself change before and after the chemical reaction?
- Will not change! In fact, the catalyst in the chemical reaction is to participate in the reaction, but in the reaction it not only participate in the reaction, but also generated, and the amount of reaction and the amount of equal, the total amount of the same, this process is more complex, But sometimes the problem will appear, but the information will be very clear, will not affect the problem!
- Q: In biology, the enzyme seems to be a tool for opening a reaction, such as the decomposition of cellulose, such as linked RNA and protein, no enzyme can not. But in chemistry, the catalyst is only a regulatory role, change the reaction rate only. The teacher said that the enzyme is the catalyst. Is there any other effect of the enzyme? (Ignorant high three dogs, you do not spray the big god)
- But let's not say that there is no enzyme or catalyst, the reaction can not be carried out. In the process of random collision of a molecule, the occasional jump of 3 meters is also possible, but the probability is low. From the macro point of view, is the reaction rate is low. So what rate, catalyst, or what xxx let high school teacher how to say, chemical catalysis and enzyme catalysis of the fundamental principles are the same, so you go to college to understand.
- Q: Are the catalysts for upgrading the weapons components that i may have a few of, or very rare ones?
- catalyst's cannot be used apart from transforming items. They will be greyed out at all other times. They always say in the description can be used as a catalyst as well. btw, are you using 36 sturdy bones or vibrance ooze to get the 3x multiplyer strait up? oh yeah, never ever use any item at all that says can be sold for a premium. Always sell those items, they are worth lots of money. And when you start buying components, only ever buy particle accelerator, perfect conductor or ultra compact reactor. Perfect conductor gives least bang for $$$ out of the 3, whereas ultra compact reactor gives most bang for $$$. And the sturdy bones and vibrance ooze for multiplyer purposes. Hope this helped :)
Send your message to us
Amino Trimethylene Phosphonic Acid Manufacturer Under ISO Test
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords





















