Amino Trimethylene Phosphonic Acid SGS Test
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Product Description:
Amino tris(methylene phosphonic acid) / Amino Trimethylene Phosphonic Acid/ ATMP / 6419-19-8 / C3H12NO9P3
CAS No. 6419-19-8
Molecular Formula: N(CH2PO3H2)3
Molecular weight: 299.05
Structural Formula: 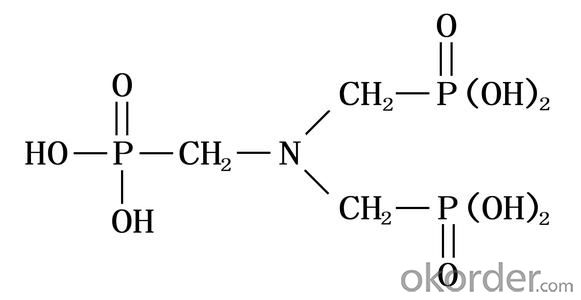
Properties:
ATMP has excellent chelation, low threshold inhibition and lattice distortion ability. It can prevent scale formation, calcium carbonate in particular, in water system. ATMP has good chemical stability and is hard to be hydrolyzed in water system. At high concentration, it has good corrosion inhibition.
ATMP is used in industrial circulating cool water system and oilfield water pipeline in fields of thermal power plant and oil refinery plant. ATMP can decrease scale formation and inhibit corrosion of metal equipment and pipeline. ATMP can be used as chelating agent in woven and dyeing industries and as metal surface treatment agent.
The solid state of ATMP is crystal powder, soluble in water, easily deliquescence, suitable for usage in winter and freezing districts. Because of its high purity, it can be used in woven & dyeing industries and as metal surface treatment agent.
Specification:
| Items | Index | |
|---|---|---|
| Standard | Solid | |
| Appearance | Clear, Colorless to pale yellow aqueous solution | White crystal powder |
| Active acid % | 50.0-51.0 | 95.0min |
| Chloride (as Cl-)% | 1.0 max | 1.0 max |
| pH value (1% solution) | 2.0 max | 2.0 max |
| Fe,mg/L | 10.0max | 20.0max |
| Density (20°C)g/cm3 | 1.31-1.35 | - |
| Colour APHA (Hazen) | 30.0max | - |
Application range&using method:
ATMP is usually used together with other organophosphoric acid, polycarboxylic acid and salt to built all organic alkaline water treatment agent. ATMP can be used in many different circulating cool water system. The recommended dosage is 5-20mg/L. As corrosion inhibitor, The recommended dosage is 20-80mg/L.
Package and Storage:
ATMP liquid: Normally In 30kg or 250kg net Plastic Drum;ATMP solid: 25kg inner liner polyethylene (PE) bag, outer plastic woven bag, or confirmed by clients request.Storage for ten months in room shady and dry place.
Safety Protection:
ATMP is Acidity, Avoid contact with eye and skin, once contacted, flush with water.
Shipping Date: Within 7-10 workdays after receiving your deposit.
Our Service:
Own Lab and joint venture factory.
Superb r&d team;Safety standardization production.
Rich experience in export and strong logistical support.
Good relationship with many large domestic pharmaceutical factory.
Perfect service, perfect supply chain.
- Q: High chemistry: Does the catalyst affect the heat and heat of the reaction?
- No
- Q: What is the principle of the catalyst? Why can change the rate of chemical reactions and their own without any change
- The catalyst is a substance that alters the reaction rate without changing the total standard Gibbs free energy of the reaction. The composition, chemical properties and quality of the catalyst itself do not change before and after the reaction; its relationship with the reaction system is as highly selective (or specific) as the relationship between the lock and the key. A catalyst does not catalyze all chemical reactions. For example, manganese dioxide catalyzes the thermal decomposition of potassium chlorate, accelerates the reaction rate, but does not necessarily have a catalytic effect on other chemical reactions. Some chemical reactions are not only the only catalyst, such as potassium chlorate can be thermally decomposed in the catalytic role of magnesium oxide, iron oxide and copper oxide, etc., potassium chlorate oxygen can also be used when the red brick powder or copper oxide as a catalyst.
- Q: What is a catalyst and how does it make a reaction go faster?
- Catalysts change the rate of the reaction (faster / slower) without being consumed (used up) in the reaction. They do this by providing a lower energy (easier) pathway from reactants to products. For example in the reaction A + B -D, we can split up the reaction into the two half-reactions below. The first describes the two reactants coming together and the second describes the product formation. A + B -AB AB -D If we add a catalyst which both A and B bond to easier than they do to each other this can increase the rate of the reaction by bringing A and B together on the catalyst. This can be represented with the half-equations below. A + B + Cat -ABCat ABCat -D + Cat
- Q: The properties of scandium
- Sci-Scandium (Sc) Basic knowledge Introduction In 1879, Swedish chemistry professor Nelson (LFNilson, 1840 ~ 1899) and Clive (PTCleve, 1840 ~ 1905) were almost simultaneously in rare mineral silica beryllium yttrium and black The mine found a new element. They named this element "Scandium" (scandium), ...
- Q: pls give one or two catalysts that are used in the industry for example:Rhodium catalyst in a catalytic converter of a car or the Iron catalyst for making ammoniaTHANKS :)
- u . s . a . of america has an excellent style of organic gas. In theory that's accessible to have an electric powered motor force a compressor to take the low stress gas to make it dense adequate to get adequate interior the vehicle to run for a mutually as (in actuality, gas stations with organic gas pumps do it precisely like that, that's no longer trucked to them, we've a pair in Dallas) the real undertaking is that, specific it will be pressurized, requiring a greater perfect tank than propane, that's a liquid interior the tank, and the tank must be a cumbersome cylinder for capability or numerous smaller cylinders area by making use of area. Getting this into the physique of the vehicle devoid of doing away with all the storage/bags area isn't ordinary. And ultimate efforts nevertheless finally end up with the miles in line with fill up being decrease than the gas which inserts in a small area.
- Q: How does catalyst aid a chemical reaction?
- A catalyst will lower the activation energy of a reaction, and hence allow it to happen faster. The actual mechanisms vary widely. Two mechanisms are: 1/ formation of intermediate compounds, which can then decompose into the catalyst and the required product 2/ provision of a large surface area for adsorption, so the reactant molecules can come into contact sooner.
- Q: Is the chemical reaction rate constant related to the amount of catalyst used?
- The decomposition of hydrogen peroxide, manganese dioxide in addition to the catalyst, but also in which it can be considered a carrier (from the surface of manganese dioxide to emit small bubbles, you should be able to see), so the amount of more than certainly can speed up the reaction.
- Q: High school stage which organic chemical reactions do not use catalyst
- Olefins, alkynes, making bromine water, potassium permanganate fade.
- Q: Is it not the rate to accelerate the addition of the catalyst to the catalyst, and that is why the balance does not move
- If the reaction before the catalyst, you can speed up the reaction rate, that is to achieve the balance required to reduce the time, but to balance the system when the same concentration
- Q: If possible can anyone give me information on the active site, substrates, products, and the energy of activation as part of the answer?Responses greatly appreciated! Thankss! 10pts to best answer!
- Catalysts help shift the equilibrium of a reaction to one that is more favorable. They allow a naturally occurring reaction that may be extremely slow to progress faster or an unfavorable reaction to proceed forward. During the process catalysts are recycled, which means that at the catalyst is the same compound in the beginning and the end of the reaction, although during intermediate steps catalysts can change conformation. Catalysts shift the equilibrium of a reaction by lowering the activation energy of a reaction, which is the energy barrier which must be overcome in order for the reaction to proceed in a desired direction. This can be achieved in several ways such as providing favorable thermodynamic conditions for a reaction or creating intermediates which react more favorably to create the products. Inside the cell a lot of chemical reactions are either too slow to proceed naturally or are simply unfavorable. Catalysts help overcome those barriers. The substrate is the part of the reaction which gets transformed into the products after binding to the active site of the protein.
Send your message to us
Amino Trimethylene Phosphonic Acid SGS Test
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords























