SDIC Aqua Products for Water Treatment Chemicals
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 17 m.t.
- Supply Capability:
- 1800 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
SDIC
Introduction:
CNBM--SDIC White powder or grain with chlorine odor . It is a strong oxidant and chlorate agent and can dissolved in water easily . Its aqueous solution assumes weak acidity and the active chlorine in its dry products lose little when it is stored for a long time at the atmospheric temperature .
SDIC Image:

SDIC Specification:
Chemical Name | Sodium Dichloroisocyanurate | |
Molecular Formula: | C3O3N3HCL2NA | |
Molecular Weight: | 220.96 | |
CAS Number: | 2893-78-9 | |
Product | 60% | 56% |
Available chlorine(%,min) | 60 | 56 |
Moisture content(% max) | 5 | 8 |
PH Value(1% solution) | 6-7 | 6-7 |
Particles Size:
Mesh | 5~8 | 8~30 | 20~40 | 20~60 |
Main usage:
this products can effectively kill various germs, fung uses and viruses, specially A&B type hepatitis viruses. It is effective on killing algae, decolorizing cleaning water or bleaching .It can be widely used for epidemic prevention, livestock farming , industry and agriculture.
Package:
50KG PLASTIC DRUMS/ FIBER DRUMS.
25KG PLASTIC DRUMS/FIBER DRUMS.
1000KG BIG BAGS.
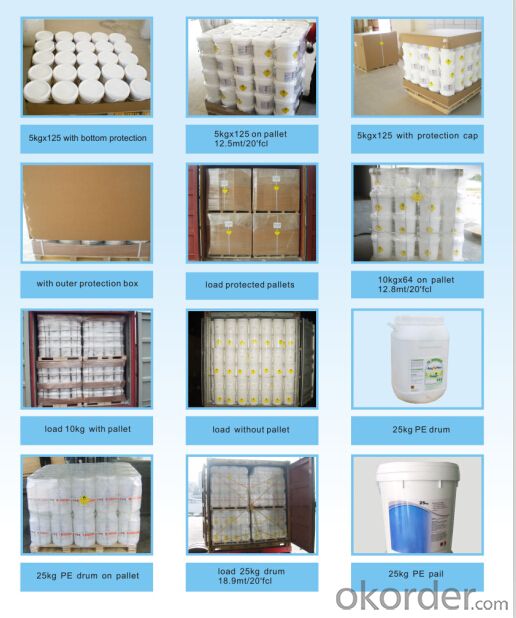
Or any other packages suggest by customers.
Service we can provide:
1. Mixed container, we can mix different items in one container.
2. Quality control, before shipment, free sample for test. after shipment, keep sample for 3 years
3. Prompt shipment with professional documents
4. Packing as your request, with photo before shipment
Our Advantages:
Full experience of large numbers containers loading in Chinese sea port
Fast shipment by reputed shipping line
Packing with pallet as buyer's special request
Best service after shipment with e mail
Cargoes together with container sales seervice available
Full experience for Canada & Japan export
Cargoes photo before and after loading into container
Raw materials from chinese origin
Professional Loading:
1.We will provide you with professional loading
2.We have one team supervise uploading the materials. We will check the container, the packages
3.Every step, taking pictures and make records.
4.we will make a complete Loading Report for our customer of each shipment.
- Q: Chemical Reactions Under what circumstances the catalyst accelerates the reaction
- First, more than ninety-nine percent of the catalyst is accelerating the reaction, and if the catalyst kinetics reduces the energy barrier of the reaction, the reaction will naturally accelerate.
- Q: What is a catalyst?
- In industrial production, the large amount of catalyst used frequently, the catalyst that does not change theoretically may sometimes change into another substance, which is the so-called poison of catalyst,
- Q: And hydrogen peroxide
- Yes, as long as it is copper ions and iron ions on the line, such as FeCI can
- Q: The size of △ H in the thermochemical reaction equation is related to the use and unused catalyst
- It does not matter
- Q: What is chemical adsorption and its relationship with heterogeneous catalysis
- The catalytic cycle includes five steps: diffusion, chemical adsorption, surface reaction, desorption and reverse diffusion.The chemical adsorption is an important part of the heterogeneous catalysis process, and the adsorption of the reactants on the catalyst surface,
- Q: Please make it simple because I need it for school and please give to examples for the second part Thanx :D
- A catalyst is a substance that speeds up the rate of a chemical reaction with itself being chemically unchanged at the end of the reaction. They are useful as they help to lower the minimum amount of energy needed ( also known as activation energy) to start the reaction. Hence, by lowering the activation energy of the reaction, they help to speed up the rate of reaction. For example, in the Haber process for the manufacture of ammonia, the catalyst iron is added to speed up the rate of reaction between hydrogen gas and nitrogen gas. Otherwise, the reaction would have proceeded much more slowly. Another example is the catalyst nickel used in the manufacture of margarine and vanadium (V) oxide for manufacturing sulfuric acid. As catalyst remain chemically unchanged after a reaction, they can be reused again and hence, they are required in minute amounts. An example is the washing powder used in washing clothes, they help to remove food stains by digesting the proteins in food. They can be reused after each reaction and hence, you do not need to add in the whole packet of washing powder but only a few spoonful.
- Q: What is the difference between an enzyme catalyst in a living body and a catalyst in chemistry?
- Enzyme has a high catalytic efficiency (high efficiency) Generally speaking, the reaction rate of the alcohol catalyzed reaction rate is 106-1013 times higher than that catalyzed by the chemical catalyst
- Q: okay im doing a project for my classroom about catalyst and i have to draw a picture but when i looked up on google i just saw a bunch of random stuff and a couple were metal so thats why im asking this question. :)
- A catalyst is something that enables a process to take place without being part of said process itself, such as in a chemical reaction.
- Q: i keep messing up on those 2 simple things haha i would apprecaite some help.
- A catalyst is a substance that affects the rate of a reaction. It may participate, but cannot be consumed in the reaction. For example, KMnO4 catalyzes the breakdown of H2O2 into H2O and O2. In the end, as much KMnO4 exists as did in the beginning. An enzyme is a biochemical reagent that allows an organism to convert a compound into other compounds. This is part of metabolic processes. For example, maltose (a sugar composed of a chain of two glucose molecules) can be broken down into glucose by the maltase enzyme. Unlike a catalyst, enzymes may or may not be consumed/altered in the metabolic processes.
- Q: Manganese dioxide can be used as a catalyst for various chemical reactions
- The reactor may be a reactant,
Send your message to us
SDIC Aqua Products for Water Treatment Chemicals
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 17 m.t.
- Supply Capability:
- 1800 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords



























