Amino Trimethylene Phosphonic Acid Wholesale Research Chemicals
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Product Description:
Amino tris(methylene phosphonic acid) / Amino Trimethylene Phosphonic Acid/ ATMP / 6419-19-8 / C3H12NO9P3
CAS No. 6419-19-8
Molecular Formula: N(CH2PO3H2)3
Molecular weight: 299.05
Structural Formula: 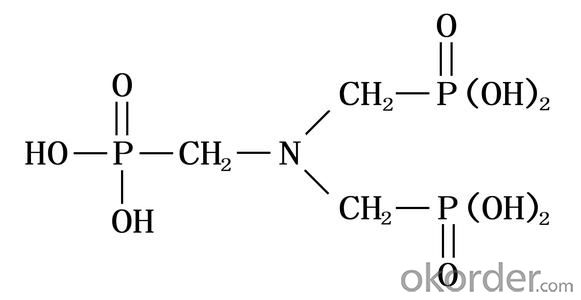
Properties:
ATMP has excellent chelation, low threshold inhibition and lattice distortion ability. It can prevent scale formation, calcium carbonate in particular, in water system. ATMP has good chemical stability and is hard to be hydrolyzed in water system. At high concentration, it has good corrosion inhibition.
ATMP is used in industrial circulating cool water system and oilfield water pipeline in fields of thermal power plant and oil refinery plant. ATMP can decrease scale formation and inhibit corrosion of metal equipment and pipeline. ATMP can be used as chelating agent in woven and dyeing industries and as metal surface treatment agent.
The solid state of ATMP is crystal powder, soluble in water, easily deliquescence, suitable for usage in winter and freezing districts. Because of its high purity, it can be used in woven & dyeing industries and as metal surface treatment agent.
Specification:
| Items | Index | |
|---|---|---|
| Standard | Solid | |
| Appearance | Clear, Colorless to pale yellow aqueous solution | White crystal powder |
| Active acid % | 50.0-51.0 | 95.0min |
| Chloride (as Cl-)% | 1.0 max | 1.0 max |
| pH value (1% solution) | 2.0 max | 2.0 max |
| Fe,mg/L | 10.0max | 20.0max |
| Density (20°C)g/cm3 | 1.31-1.35 | - |
| Colour APHA (Hazen) | 30.0max | - |
Application range&using method:
ATMP is usually used together with other organophosphoric acid, polycarboxylic acid and salt to built all organic alkaline water treatment agent. ATMP can be used in many different circulating cool water system. The recommended dosage is 5-20mg/L. As corrosion inhibitor, The recommended dosage is 20-80mg/L.
Package and Storage:
ATMP liquid: Normally In 30kg or 250kg net Plastic Drum;ATMP solid: 25kg inner liner polyethylene (PE) bag, outer plastic woven bag, or confirmed by clients request.Storage for ten months in room shady and dry place.
Safety Protection:
ATMP is Acidity, Avoid contact with eye and skin, once contacted, flush with water.
Shipping Date: Within 7-10 workdays after receiving your deposit.
Our Service:
Own Lab and joint venture factory.
Superb r&d team;Safety standardization production.
Rich experience in export and strong logistical support.
Good relationship with many large domestic pharmaceutical factory.
Perfect service, perfect supply chain.
- Q: Chemical Reactions Under what circumstances the catalyst accelerates the reaction
- First, more than ninety-nine percent of the catalyst is accelerating the reaction, and if the catalyst kinetics reduces the energy barrier of the reaction, the reaction will naturally accelerate.
- Q: Exemplify the use of green catalysts in green chemistry
- Photocatalytic water generates oxygen and hydrogen
- Q: How are the 4 characteristics of a catalyst (1. organic or inorganic 2. reusable 3. Highly specific, and 4. lowers activation energy) important in preforming life functions? please be as specific as possible, i understand that these are characteristic, i just don't understand why they're beneficial, other than the reusable and lowers activation energy one.
- Organic or Inorganic - the catalyst (enzyme) must be organic to be found in the cell. Catalysts speed up chemical reactions inside a cell and must therefore be organic to be a functioning part of the cell. Reusable - There are so many reactions that catalysts are involved in that it would be a waste for the cell if a catalyst could only last one reaction, especially if there are inhibitors and competition for the active site. Catalysts must be reusable in order to keep the cell functioning. Catalysts always remain unchanged after a reaction. HIihly Specific - Catalysts are only made to catalyze one specific chemical reaction. Their active site has proteins bonded in such a way that only certain elements can enter the active site and H bond with those proteins. The fact that they are highly specific maximizes the productiveness of the cell. And it ensures that the cell only has catalysts to reactions that it needs to be completed. It also ensures that the elements are correctly bonded with eachother. If any two elements could enter the active site, there is no guarantee that the correct product will be produced. Catalysts and Enzymes must be super highly specific in order to properly function. Lowers Activation Energy - The more energy a cell has to spend to catalye a reaction, the worse it is for the cell and the less ATP is has for other reactions. Catalyts hold the substrates together so there is less energy that is needed to have the two substrates react with eachother. Activation Energy is the energy that is needed to start a reaction. So the less energy used by the cell for reactions, the better for the cell. Hope this helps
- Q: What is a catalyst?
- The catalyst itself reduces the energy barrier (Ea, activation energy) of the chemical reaction, making the reaction easier.
- Q: 1. Catalysts can help to bring the reactants together in the correct orientation2. The chemical formula of a catalyst is written on the left hand side (reactant) side of an equation.3. Catalysts can provide a surface on which the reaction occurs.4. Catalysts increase the activation energy.5. Catalysts increase the magnitude of the equilibrium constant, thus favoring product formation.6. "Enzymes" are biochemical catalysts.7. Catalysts increase the rate of a reaction.8. Catalysts are slowly used up during the reaction and need to be replaced.
- 7 is definetly true
- Q: Chemical equation if there is a catalyst and heating, which write in the equal sign above, which written in the following? Tomorrow academic level test, solution
- At the same time, the catalyst is written on, and the heating symbol is written under the equal sign. Only one is written on the equal sign
- Q: okay im doing a project for my classroom about catalyst and i have to draw a picture but when i looked up on google i just saw a bunch of random stuff and a couple were metal so thats why im asking this question. :)
- a okorder /...
- Q: When you write a chemical equation, how do you want to add "catalyst" and "?" When you do not have to write?
- This is the need for your memory, write a few times, will naturally cooked
- Q: give an example of how a catalysts speeds up the rate of reaction?? thank you!!?
- When making margarine, nickel is used to speed up the process.
Send your message to us
Amino Trimethylene Phosphonic Acid Wholesale Research Chemicals
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords






















