Amino Trimethylene Phosphonic Acid Best Quality
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Product Description:
Amino tris(methylene phosphonic acid) / Amino Trimethylene Phosphonic Acid/ ATMP / 6419-19-8 / C3H12NO9P3
CAS No. 6419-19-8
Molecular Formula: N(CH2PO3H2)3
Molecular weight: 299.05
Structural Formula: 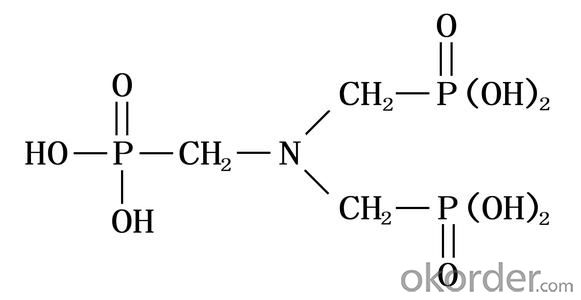
Properties:
ATMP has excellent chelation, low threshold inhibition and lattice distortion ability. It can prevent scale formation, calcium carbonate in particular, in water system. ATMP has good chemical stability and is hard to be hydrolyzed in water system. At high concentration, it has good corrosion inhibition.
ATMP is used in industrial circulating cool water system and oilfield water pipeline in fields of thermal power plant and oil refinery plant. ATMP can decrease scale formation and inhibit corrosion of metal equipment and pipeline. ATMP can be used as chelating agent in woven and dyeing industries and as metal surface treatment agent.
The solid state of ATMP is crystal powder, soluble in water, easily deliquescence, suitable for usage in winter and freezing districts. Because of its high purity, it can be used in woven & dyeing industries and as metal surface treatment agent.
Specification:
| Items | Index | |
|---|---|---|
| Standard | Solid | |
| Appearance | Clear, Colorless to pale yellow aqueous solution | White crystal powder |
| Active acid % | 50.0-51.0 | 95.0min |
| Chloride (as Cl-)% | 1.0 max | 1.0 max |
| pH value (1% solution) | 2.0 max | 2.0 max |
| Fe,mg/L | 10.0max | 20.0max |
| Density (20°C)g/cm3 | 1.31-1.35 | - |
| Colour APHA (Hazen) | 30.0max | - |
Application range&using method:
ATMP is usually used together with other organophosphoric acid, polycarboxylic acid and salt to built all organic alkaline water treatment agent. ATMP can be used in many different circulating cool water system. The recommended dosage is 5-20mg/L. As corrosion inhibitor, The recommended dosage is 20-80mg/L.
Package and Storage:
ATMP liquid: Normally In 30kg or 250kg net Plastic Drum;ATMP solid: 25kg inner liner polyethylene (PE) bag, outer plastic woven bag, or confirmed by clients request.Storage for ten months in room shady and dry place.
Safety Protection:
ATMP is Acidity, Avoid contact with eye and skin, once contacted, flush with water.
Shipping Date: Within 7-10 workdays after receiving your deposit.
Our Service:
Own Lab and joint venture factory.
Superb r&d team;Safety standardization production.
Rich experience in export and strong logistical support.
Good relationship with many large domestic pharmaceutical factory.
Perfect service, perfect supply chain.
- Q: High school chemistry, catalyst activation energy map
- Catalytic reaction is the reaction of the first reaction with the catalyst or attached to the catalyst to form intermediates, and then further reaction to produce products and catalysts, so the amount of catalyst in theory is the same! The activation of these two processes can be reduced! So there will be two peaks! Can be simplified as a peak!
- Q: Characteristics and types of catalysts?
- Catalysts don't undergo any change. and types of catalysts - 1) Homogeneous Catalysts ( Having same phase that of reactant, product i.e. reactant and product and catalysts all are either liquid or gas or solid.). 2) Hetrogenrous Catalysts (Different Phase than that of reactant and product. 3) Autocatalysts (reaction proceed catalysed as product is formed or product catalyse the reaction.)
- Q: Chemical questions: "CO2 and H2 in the catalyst conditions have a reaction, the reaction of the chemical equation is"
- CO2 + H2 = CO + H2O (conditions: catalyst, generally requires heating, and reversible)
- Q: What happens to a catalyst after a chemical reaction?
- Only a catalyst? Poor catalyst. Catalysts get very little respect. Folks assume that catalysts don' do anything, yet they magically speed up a reaction without taking part in the reaction. That just isn't the case. Most chemical reactions take place in multiple steps. A catalyst can be a reactant in one step and a product in s subsequent step, thereby giving the impression that it did not react. The catalyst speeds up a chemical reaction by providing an alternate reaction pathway which has a lower activation energy. The lower activation energy means that more molecules will have the energy required to react, and the rate will be greater. So the bottom line is that the catalyst will have appeared not to have reacted, and returns to its original state.
- Q: Can manganese dioxide do any catalyst for chemical reactions?
- The catalyst is involved in the reaction during the reaction and is reduced to the original composition after the reaction is completed. For example: heating decomposition of potassium permanganate when adding potassium permanganate. Potassium permanganate decomposition process, potassium permanganate is involved in the reaction, the specific way is not clear. And finally appeared with potassium permanganate. Reaction before and after the catalyst morphology changes, particles become powder, powder particles and so on.
- Q: Will the catalyst change in the chemical reaction?
- The catalyst is actually involved in the chemical reaction, the catalyst is added to the reaction, becomes the other material, and then the reaction becomes back, and appears to have no change, actually involved in the change, but the end result the catalyst did not change
- Q: I was hoping to buy a land rover lr4 or lr2, but with the lr4 having gas mileage in the mid teens, i wanted to know if there is a way to improve it. I dont drive on the highway too much. I'd like to know if there is anything else to improve mileage too. I drive a lot of people around for functions, family, and others and I looked at other suvs but those two looked the best.
- Fuel Catalyst
- Q: What is the difference between biological and chemical catalysts?
- catalysts which catalyze the chemical reactions taking place in human body such as enzymes are called bio-catalyst and other one's which are generally used in laboratory by chemists for multiple type of reactions are chemical ctalysts
- Q: Will the catalyst be able to increase the rate of chemical reactions?
- Not necessarily, but junior high school, such as manganese dioxide is a positive catalyst, that is, "to speed up the reaction rate", when the teacher should also mentioned "to reduce the rate of response" situation
- Q: how can you tell when a substance serves as a catalyst?
- It makes a reaction run faster and better AND it is not used up by the reaction
Send your message to us
Amino Trimethylene Phosphonic Acid Best Quality
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches



















