Amino Trimethylene Phosphonic Acid
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Product Description:
Amino tris(methylene phosphonic acid) / Amino Trimethylene Phosphonic Acid/ ATMP / 6419-19-8 / C3H12NO9P3
CAS No. 6419-19-8
Molecular Formula: N(CH2PO3H2)3
Molecular weight: 299.05
Structural Formula: 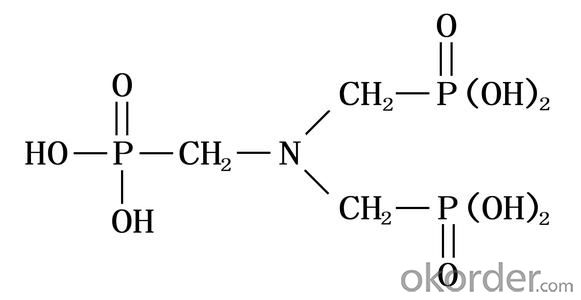
Properties:
ATMP has excellent chelation, low threshold inhibition and lattice distortion ability. It can prevent scale formation, calcium carbonate in particular, in water system. ATMP has good chemical stability and is hard to be hydrolyzed in water system. At high concentration, it has good corrosion inhibition.
ATMP is used in industrial circulating cool water system and oilfield water pipeline in fields of thermal power plant and oil refinery plant. ATMP can decrease scale formation and inhibit corrosion of metal equipment and pipeline. ATMP can be used as chelating agent in woven and dyeing industries and as metal surface treatment agent.
The solid state of ATMP is crystal powder, soluble in water, easily deliquescence, suitable for usage in winter and freezing districts. Because of its high purity, it can be used in woven & dyeing industries and as metal surface treatment agent.
Specification:
| Items | Index | |
|---|---|---|
| Standard | Solid | |
| Appearance | Clear, Colorless to pale yellow aqueous solution | White crystal powder |
| Active acid % | 50.0-51.0 | 95.0min |
| Chloride (as Cl-)% | 1.0 max | 1.0 max |
| pH value (1% solution) | 2.0 max | 2.0 max |
| Fe,mg/L | 10.0max | 20.0max |
| Density (20°C)g/cm3 | 1.31-1.35 | - |
| Colour APHA (Hazen) | 30.0max | - |
Application range&using method:
ATMP is usually used together with other organophosphoric acid, polycarboxylic acid and salt to built all organic alkaline water treatment agent. ATMP can be used in many different circulating cool water system. The recommended dosage is 5-20mg/L. As corrosion inhibitor, The recommended dosage is 20-80mg/L.
Package and Storage:
ATMP liquid: Normally In 30kg or 250kg net Plastic Drum;ATMP solid: 25kg inner liner polyethylene (PE) bag, outer plastic woven bag, or confirmed by clients request.Storage for ten months in room shady and dry place.
Safety Protection:
ATMP is Acidity, Avoid contact with eye and skin, once contacted, flush with water.
Shipping Date: Within 7-10 workdays after receiving your deposit.
Our Service:
Own Lab and joint venture factory.
Superb r&d team;Safety standardization production.
Rich experience in export and strong logistical support.
Good relationship with many large domestic pharmaceutical factory.
Perfect service, perfect supply chain.
- Q: What is the principle of catalyst reaction rate in chemical reactions?
- Whether the chemical reaction can be carried out according to the change of free energy, but only according to the change of free energy can not determine whether the reaction can be completed, because the chemical reaction is also completed by the reaction of the energy barrier, that is, if the reaction energy barrier is high, To provide some energy, across the barrier, to complete the reaction. The energy barrier is called activation energy. And the role of the catalyst is to reduce the activation energy, so that in a relatively harsh environment, chemical reaction occurs.
- Q: Is the catalyst used in the starch phosphate reaction
- (Cat1, cat2, cat4 and cat5) in the presence of terephthalic acid,
- Q: What is the chemical nature of the enzyme?
- The difference between the enzyme and the general protein is that the enzyme is a protein with a special catalytic function. Similarly, the enzyme, like other proteins, consists mainly of amino acids, with one, two, three and quaternary structures, and the same enzyme as other proteins The composition of the enzyme can be divided into two types: simple protein and binding protein. Some enzymes are only protein, its activity depends on its protein structure, such enzymes are simple protein; other enzyme active ingredients in addition to containing protein, but also There are some small molecules that cofactor, the two together to be active, such enzymes belong to the binding protein.The protein part of the protein is called the enzyme protein, non-protein part called the cofactor
- Q: What is the catalyst condition in the chemical equation?
- On the middle of the equal sign or arrow above ah ~
- Q: What are the properties of the catalyst (eg, specificity)?
- The definition of a chemical reaction in the chemical reaction can change the chemical reaction rate of other substances, and its quality and chemical properties before and after the reaction did not change the material called catalyst, also known as catalyst. Catalyst in the role of chemical reaction There is also a saying that the catalyst reacts first with one of the reactants and then the two products continue to undergo a new chemical reaction under the original conditions and the reaction conditions of the catalyst reaction are more reactive than the original reaction The reaction conditions of the catalyst have been changed by the reaction of the catalyst by the reaction of the catalyst, that is, the quality and chemical properties mentioned above did not change before and after the reaction.
- Q: Does increasing the amount of catalyst added to, say, a solution of Hydrogen Peroxide, make the rate of reaction go faster. Is the rate of reaction directly proportional to the amount of catalyst added to the solution? Or does the experiment go at the same rate regardless of how much catalyst there is? Thanks would really appreciate some answers. - Sarah
- Adding a catalyst would increase the rate of reaction. This could decrease the activation energy, the amount of kinetic energy needed for the reaction to occur. Hope that helps
- Q: What are the catalysts?
- The relationship between it and the reaction system is as highly selective (or specific) as the relationship with the key. A catalyst does not catalyze all chemical reactions, such as manganese dioxide in the decomposition of potassium chlorate Play a catalytic role to speed up the chemical reaction rate, but the other chemical reactions do not necessarily have a catalytic effect
- Q: What is the analytical principle of chemical adsorbents? How about the number of active catalyst centers tested?
- What do you mean by the chemical adsorber? BET is the use of the surface of the uneven force field, but the inert gas at low temperature in the surface adsorption. TPD, TPR is the number of active centers that can be measured by the technique of desorption and reduction between specific gases and catalysts as the temperature increases. If the active site is a reduced position, H2-TPR can be used. If the active site is acidic, NH3-TPD can be used, but also the method of alkali titration.
- Q: Why would the Eact decrease if a catalyst is added?
- Catalysts work by providing an (alternative) mechanism involving a different transition state and lower activation energy. The effect of this is that more molecular collisions have the energy needed to reach the transition state. Hence, catalysts can perform reactions that, albeit thermodynamically feasible, would not run without the presence of a catalyst, or perform them much faster, more specific, or at lower temperatures. This can be observed on a Boltzmann distribution and energy profile diagram. This means that catalysts reduce the amount of energy needed to start a chemical reaction.
Send your message to us
Amino Trimethylene Phosphonic Acid
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords





















