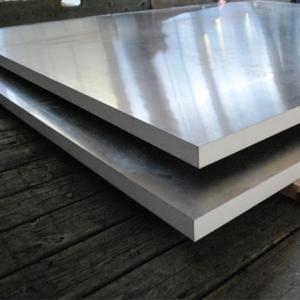
Gold Aluminum Foil Sheets - 5083 Aluminium Alloy Plate for Marine/Aluminium Cast Plates 5083 5052
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 20000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Specification
Aluminum Sheet for Making Aluminum Composite Panel
Aluminum sheet specifications:
1) Alloy : 1050 1060 1070 1100 2024 3003 3004 3105 3A21 5005 5052 5083 5754 5182 5454 5456 6061 6063 7075 8011 etc
2) Temper: O/H12/H14/H1/H18/H32/H34/H36/H38//H111/H112/H116/H321/T6/T651/T3/T351 etc
3) Thickness: 0.1mm to 300mm
4) Width:20mm to 3300mm
5)Length: ≤ 12000mm
6) Protective film can be added
7) Production Line: DC and CC production line
Discription:
Width: 50-800mm
Thickness: 8-25mic
Length: 3-300m
Character:
High-temperature sterilization
Made of advanced food grade alu material,no pollution
Eco-friendly, recoverable and recyclable
Application:
widely used for food cooking, freezing wrapping, storing etc, the main application are for household and catering, which are targeting for retail market and food service market, such as hypermarket, chain store and hotel, restaurant etc.
Packing:
One roll in an individual box or printed PP bag
Certain quantities in a standard carton
Depends on customer’s request
Service
1. We have the good and professional team, have a good after-sales service.
2. Accept any drawings or your samples to develop new product.
3. Offer relateive technical support,quick response,all your inquire will replied within 24 hours.
4. OEM, buyer design, buyer label services provided.
5. Have the certification of ISO 9001, SGS.
6. Special discount and protection of sales area provided to our distributor.
FAQ
Q1:Do you provide sample? How many days will samples be finished?
Free samples will be provided if needed, but customers should undertake shipping cost or freight charges, samples will be finished in 5-7days
Q2: Can we visit your factory?
Welcome to our factory at any time.
Q3: Complaint solving process
Finding your salesman—Salesman provide you the solution (If it’s our responsibility, we will resend substitutes or return money or provide discount for your next order, etc.; If it’s shipping company’s responsibility, we will also help you until the problem is resolved.) —If salesman can’t solve your problem, please call our manager .
Q4: Delivery time
3~30working days after confirming the payment. If the order is urgent, we will push our workers to finish in advance.
Q5: What's your MOQ?
Normally 8MT are requested as the minium order quantity ,we shall give
additional instructions in special circumstances.
Q6: What are the terms of payment and currencies do you accept?
T/T or L/C is accepted, currently we appreciated your payment through
USD,EUR, RMB
Q7: Do you accept customized orders?
Yes, we do. Your customized orders are always welcomed. Please kindly offer us your samples or drawings, so that we can customize the products according to your preferences. About any further detail, please feel free to contact us.
Q8: What information should I let you know if I want to get a quotation?
Your detailed requirements regarding the products's dimensions, including shape, thickness, top out (length*width*height), and your order quantity are highly appreciated if you want further information about our quotation
Q9: How about the mass production?
The lead time of mass production depend on quantity, usually 25-30days (20FT) .





- Q: (1) Aluminum is malleable. (2) Aluminum reacts with sulfuric acid.(3) Aluminum conducts an electric current.(4)Aluminum has a density of 2.698 g/cm3 at STP.Explain why.
- The answer is choice 2. Why? It's the only one that describes a chemical interaction. The other three describe physical properties of aluminum, that is properties that do not involve a change in the chemical identity of aluminum metal. In choices 1, 3, and 4, the aluminum metal remains as aluminum metal. However, in choice 2, the metal reacts and becomes aluminum ion.
- Q: This question asks about the various colors and finishes that can be applied to aluminum sheets.
- <p>Aluminum sheets are available in a wide range of colors and finishes, offering versatility for various applications. Common colors include silver, white, black, and bronze, but they can also be anodized or painted to achieve virtually any color. Finishes can be smooth, brushed, or embossed, with options like matte, satin, and gloss. Additionally, there are specialty finishes like mirror-like polished, hammered, and wood grain textures. The choice of color and finish depends on the specific requirements of the project, such as aesthetics, durability, and resistance to environmental factors.</p>
- Q: how to process 0.3mm hole on aluminum material?
- It's bored by drill.
- Q: Can aluminum sheet be used for heat sinks?
- Indeed, heat sinks can utilize aluminum sheet as a viable option. Aluminum, known for its commendable thermal conductivity attributes, is frequently employed as a material for heat sinks. Its lightweight nature, cost-effectiveness, and resistance to corrosion contribute to its popularity. With the ability to easily mold aluminum sheet into diverse shapes and sizes, it becomes suitable for a range of heat sink designs. The flat and thin composition of aluminum sheet aids in effective heat dissipation by expanding the available surface area for heat transfer. Moreover, aluminum can be readily machined or extruded to produce fins, further enhancing the heat dissipation capabilities of the heat sink. Overall, due to its myriad advantages, aluminum sheet stands as a favored choice for heat sinks in a multitude of applications, encompassing electronics, computers, LED lights, and automotive components.
- Q: This question asks for a list of various adhesive types that are suitable for bonding aluminum sheets together.
- <p>There are several types of adhesives used for bonding aluminum sheets, including epoxy adhesives, which are strong and resistant to heat and chemicals; acrylic adhesives, known for their flexibility and UV resistance; cyanoacrylate or super glue, which offers fast bonding; polyurethane adhesives, providing excellent adhesion and durability; and silicone adhesives, which are great for high-temperature applications. Each type has its specific properties and applications, making them suitable for different bonding requirements and environmental conditions.</p>
- Q: Can 101 aluminum sheets be painted or coated?
- Certainly! Aluminum sheets offer the possibility of being painted or coated. This adaptable material lends itself to receiving a range of finishes, including paint or protective coating, which can both elevate its visual appeal and safeguard it against corrosion. Whether it pertains to 101 aluminum sheets or any other aluminum variant, diverse techniques like spray painting, powder coating, or anodizing can be employed to paint or coat them. The selection of the appropriate paint or coating shall be contingent upon the desired aesthetics, performance criteria, and the precise utilization of the aluminum sheets.
- Q: Are the aluminum sheets suitable for manufacturing sporting equipment?
- Aluminum sheets prove to be a suitable choice for the production of sporting equipment. This is due to aluminum being a material that is both lightweight and durable, offering various advantages in the manufacturing process. Its exceptional strength-to-weight ratio makes it highly suitable for applications where strength is necessary, while still keeping the overall weight of the equipment low. Moreover, aluminum possesses a high level of resistance to corrosion, a crucial quality for sporting equipment that is frequently exposed to diverse weather conditions. In addition to this, aluminum can be easily molded into different shapes and sizes, granting manufacturers the ability to create personalized equipment for a range of sports. In summary, utilizing aluminum sheets in the manufacturing of sporting equipment guarantees the creation of top-quality, lightweight, and long-lasting products that enhance performance and durability.
- Q: How are aluminum sheets measured and specified?
- Aluminum sheets are typically measured and specified based on their thickness, width, and length. The thickness is commonly expressed in gauge or millimeters, while the width and length are provided in inches or millimeters. Additionally, the alloy composition and temper of the aluminum sheet may also be specified to meet specific requirements.
- Q: Are aluminum sheets suitable for solar reflectors?
- Yes, aluminum sheets are suitable for solar reflectors. Aluminum is widely used in the construction of solar reflectors due to its high reflectivity and durability. Its reflective properties allow it to efficiently redirect sunlight onto a solar panel or other solar devices, optimizing energy absorption. Additionally, aluminum is lightweight and corrosion-resistant, making it an ideal material for outdoor applications. Its affordability and ease of fabrication also contribute to its popularity in the solar industry.
- Q: Can 101 aluminum sheets be used in electrical or electronic components?
- Yes, 101 aluminum sheets can be used in electrical or electronic components.
Send your message to us
Gold Aluminum Foil Sheets - 5083 Aluminium Alloy Plate for Marine/Aluminium Cast Plates 5083 5052
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 20000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords