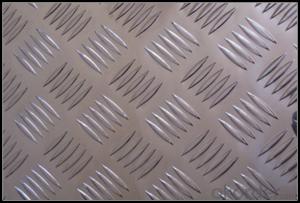
Aluminum Foil Sheets Five Bar Treadplate Aluminium Panel for Tool Box
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 10000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Specification
1. Specification of Five Bar Treadplate Aluminium Panel for Tool Box
Alloy Number | AA1XXX,AA3XXX,AA5XXX |
Temper | H12, H14, H16, H18, H22, H24, H26, H32, HO, F |
Thickness | 0.1mm – 500mm |
Width | 10mm- 2200mm |
Standard | GB/T3880-2006, ASTM, ISO, EU standard |
2. Application of Five Bar Treadplate Aluminium Panel for Tool Box
Transfporation, vehicle, antiskid plate,toolbox,canopy body,
3. Feature of Five Bar Treadplate Aluminium Panel for Tool Box
Surfact Quality :
Be free from Oil Stain, Dent, Inclusion, Scratches, Stain, Oxide Dicoloration, Breaks, Corrosion, Roll Marks, Dirt Streaks and other defect which will interfere with use,
Mechenical Property:
Chemical Composite and Mechanical Property
4. Certificate:
SGS and ROHS(if client request, paid by client), MTC(plant provided), Certificate of Origin(FORM A, FORM E, CO), Bureau Veritas and SGS (if client request, paid by client), CIQS certificate
5. Image of Five Bar Treadplate Aluminium Panel for Tool Box




6. Package and shipping of Five Bar Treadplate Aluminium Panel for Tool Box
First, plastic cloth with drying agent inside; Second, Pearl Wool ; Third, wooden cases with dry agent , fumigation wooden pallets, aluminum surface could cover blue PVC film
7. FAQ
1) What is the delivery time?
Dpends on actual order, around 20 to 35 days
2)What is the QC system:
We have QC staff of 20 persons and advanced equipment, each production is with MTC traced from Aluminum ingot lot.
3) What market do you mainly sell to?
Australia, America, Asia, Middle East, Western Europe, Africa etc
- Q: A 0.250-g sample of a magnesium-aluminum alloy dissolves completely in an excess of HCl (aq). When the liberated H2 is collected over water at 29 C and 752 torr, the volume is found to be 311 mL. The vapor pressure of water at 29 C is 30.0 torr. What is the mass percentage of aluminum in this alloy?
- P_H2 = 0.950 atm (Dalton's Law of partial pressures) n=Pv/RT = (0.950 atm)(0.311 L) / (0.08206 (L*atm)/(mol*K))(302.15 K) n_H2 = 0.011915983 mol Balanced equations: Al + 3HCl -- 3/2H2 + AlCl3 Mg + 2HCl -- H2 + MgCl2 By these equations, we know that every mole of Al will give us 1.5 moles of H2, and every mole of Mg will give 1 mole of H2. We can therefore set up an equation for the mass of Al like this: *Let a = the mass of MAGNESIUM* Al = 0.250 g - a With this equation in mind, we can setup two equations solving for 'n' of each element by dividing by its molar mass and multiplying by the molar ratio: n_Mg = a / 24.30 (1:1 ratio, so we don't have to multiply) -- number of moles of H2 produced by the reaction of Mg (now written as n_H') = a / 24.30 n_Al = (0.250 g - a) / (26.98 g/mol) Because of the molar ratio shown above, we must multiply n_Al by 1.5 in order to get n_H2 produced by the reaction of aluminum, hereafter known as n_H2 Since we know the number of moles produced by the sum of the reactions, we can add these equations together and solve for n_H2. (**note that your value will be different because you have a different volume**) Set up the equation like this: n_H2' + n_H2 = n_H2 = 0.011915983 mol Sub in your individual equations for n_H2' and n_H2: (a/24.3) + 1.5[(0.250-a)/26.98] = 0.011915983 mol Rearrange and solve for a (mass of MAGNESIUM): (26.98a + 9.1125 - 36.45a) / (24.3)(26.98) = 0.011915983 0.011915983 = 9.47a a = 0.137298281 g Once you have your 'a' value, divide it by the total mass (0.250 g) and multiply by 100%. This gives you the percentage of Mg. (0.137298281 g / 0.250 g) * 100% = 54.9193 % Since you want ALUMINUM, you must subtract the percentage of Mg from 100. 100 - 54.9193 = 45.08% So, the mass percentage of aluminum is 45.08%. I hope this is helpful!
- Q: My understanding of the periodic table, the transitional metals all rust because the S shells are higher energy then the D shells. So all transitional metals have 2 valence electrons. (Roughly...some electrons like to move around and give different apparent charges.) So why does aluminum corrode if it doesn't have a 2+ charge?
- 'Rusting' commonly refers to the corrosion (oxidation) of iron so when talking about other metals, it is better to use the term 'corrosion' or 'oxidation'. Aluminum can corrode and the fact that it has a general oxidation number of +3 doesn't really matter. Many elements which have a charge that is different from +2 can oxidize. Alkali metals for instance (which have a charge of +1) can oxidize. Lithium can form lithium oxide (Li2O), sodium can form sodium oxide (Na2O) and so on. However, aluminum is known to be quite resistant to corrosion (oxidation) because it spontaneously forms a thin (solid) oxide layer at it's surface protecting it from further oxidation whereas iron, for an example, will easily lose that thin layer (it ''peels off easily'') exposing more iron to corrosion. So since Al has a +3 charge and O has a -2 charge, you'll need 2 atoms of Al and 3 atoms of O to make an electrically neutral compound. 2 atoms of Al = +6 charge 3 atoms of O = -6 charge Hence Al2O3 which is aluminum oxide. I hope it helps.
- Q: Can aluminum sheets be perforated?
- Indeed, it is possible to perforate aluminum sheets. Perforating entails either punching holes or generating a pattern of holes in a material. Aluminum, being a flexible and adaptable metal, can be easily perforated through a variety of techniques like punching, drilling, or laser cutting. The perforation of aluminum sheets can have numerous applications, including facilitating airflow, reducing weight, improving aesthetics, or constructing filtration systems. The dimensions, form, and layout of the perforations can be tailored to satisfy particular demands and design preferences. In summary, perforating aluminum sheets can effectively enhance their functionality and visual allure.
- Q: Can aluminum sheets be used for HVAC systems?
- Yes, aluminum sheets can be used for HVAC systems. Aluminum is a popular material choice for HVAC applications due to its many advantageous properties. It is lightweight, making it easier to handle and install. Aluminum is also highly resistant to corrosion, which is crucial for HVAC systems that are exposed to moisture and varying temperatures. Additionally, aluminum has excellent thermal conductivity, allowing for efficient heat transfer. This makes it ideal for heat exchangers and other components in HVAC systems. Overall, aluminum sheets are a reliable and durable option for HVAC systems.
- Q: How do aluminum sheets compare to steel sheets in terms of weight?
- In terms of weight, aluminum sheets are much lighter than steel sheets. The reason for this is that aluminum has a lower density, making it a more versatile and lightweight material compared to steel. The extent of the weight difference between aluminum and steel sheets will vary depending on the thickness and dimensions of the sheets. However, as a general rule, aluminum sheets can weigh approximately 1/3 less than steel sheets of the same dimensions. This quality makes aluminum sheets particularly suitable for industries like aerospace, where weight is a crucial consideration, as well as for lightweight structures.
- Q: How do you prevent oxidation of aluminum sheets?
- To avoid oxidation of aluminum sheets, several techniques can be utilized. One commonly used method is to apply a protective coating or finish on the surface of the aluminum sheets. This coating acts as a barrier between the aluminum and the external environment, preventing the metal from reacting with oxygen and forming an oxide layer. There are various types of coatings available for this purpose, such as anodizing, painting, and powder coating. Anodizing involves creating a controlled oxide layer on the aluminum surface through electrolysis. This oxide layer is highly resistant to corrosion and provides excellent protection against oxidation. Painting and powder coating, on the other hand, involve applying a layer of paint or powdered polymer to the aluminum surface. These coatings create a physical barrier that shields the metal from oxygen and moisture. Another effective approach to prevent oxidation is by using aluminum alloys that have enhanced corrosion resistance. These alloys are specifically designed to have a higher resistance to oxidation and can withstand exposure to harsh environments without forming a significant oxide layer. In addition to coatings and alloy selection, proper storage and handling practices play a crucial role in preventing oxidation. It is important to store aluminum sheets in a clean, dry, and well-ventilated area to minimize exposure to moisture and corrosive elements. Contact with acidic or alkaline substances should be avoided, as they can accelerate the oxidation process. Regular cleaning and maintenance of aluminum sheets also help prevent oxidation by removing any contaminants that could promote corrosion. In summary, preventing oxidation of aluminum sheets involves a combination of protective coatings, appropriate alloy selection, and proper storage and handling practices. By implementing these measures, the lifespan and durability of aluminum sheets can be significantly improved.
- Q: Can the aluminum sheets be used for manufacturing chemical reaction vessels?
- Yes, aluminum sheets can be used for manufacturing chemical reaction vessels.
- Q: I did electrolysis by adding aluminum to the ends of the wire. I waited about 2 hours, and then I filtered the water. After it dried, I was left with powder. It's gray.It that aluminum powder or something else? I'm making thermite, if its not aluminum powder will it still work?
- Kinda sorta, the problem with this is that all you did was made aluminium oxide or hydroxide, when you electrolyzed the aluminium you also electrolyzed a bit of water with it, which added a hydroxyl group to the aluminum, which may have dropped a hydrogen when you dried it. If you could find an aluminium compound that is water soluble (DAMNED HARD to find) you could electrolyze it in water to get aluminium dust in the water, but that isnt very reliable or economical, the best thing for you to do is to get a ball mill or rock tumbler, add aluminium fold and about half full of regular marbles, let it run for 3 or so weeks. You need it really fine!
- Q: why intact aluminum sheet stop quicker than pectinate one in the magnetic field?
- while swinging in the magnetic field, intact aluminum sheets will formulate inner eddy current,that is the annular induced current.eddy current will transform the mechanical energy into heat energy, which makes the aluminum sheet stop quickly.but pectinate aluminum sheet can't formulate intact eddy current, so intact aluminum sheet stop quicker than pectinate one.
- Q: Is it possible for an individual to install their own aluminum sheet roofing?
- <p>Yes, you can install your own aluminum sheets roof, but it requires some skills and knowledge. You'll need to measure and cut the sheets accurately, secure them properly, and ensure watertight installation. It's advisable to have experience in roofing or construction, or to follow detailed instructions and safety precautions. For complex roofs or if you're unsure, hiring a professional is recommended to avoid damage or injury.</p>
Send your message to us
Aluminum Foil Sheets Five Bar Treadplate Aluminium Panel for Tool Box
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 10000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords