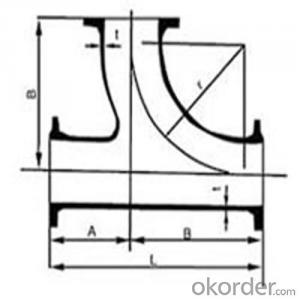
ductile iron double flanged reducer
- Loading Port:
- China Main Port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Specifications
ductile iron puddle flanged pipe
1.DN50-DN2000mm
2.EN545 OR ISO2531
3.Epoxy coating or bitumen+zinc coating
4.PN10/PN16
ductile iron pipe fittings
Standard: ISO2531 BS/EN545
Material: Ductile Iron or other Ductile Iron
Coating: epoxy in blue,black or red. or bitumen +zinc
Packing: standard Wooden pallets or cases.
Ductile iron pipe fittings type:
Flange type: double flanged bend,all flanged tee,flanged bell mouth,all flanged cross, double flanged taper,flanged spigot,blank flange,double flanged 90 long radius bend,double flanged 90 ductfoot bend.loose flanges,all flanged 45 angle branches,all flanged Y pipes, all flanged tee,double flanged pipe,flanged spigot.
Gaskets :EPDM
- Q: The following have all been scratched to expose the iron.1. An iron strip coated in plastic2.An iron strip coated in zinc3. An iron strip coated in tin4. An iron strip coated in paintAll are in a salt solution - which would rust quickly?
- you upload weight while it rusts by way of fact which you upload O2. once you scrape off rust you do away with each and all the O2 and multiple of the Fe additionally. So now the scraped iron weighs decrease than it did initially. To get a numerical answer you may desire to be attentive to precisely what sort of iron oxide you receive.
- Q: okay i no when somebody has not enough iron they tend to have nose bleeds, but when u have too much iron. what happens?
- Iron toxicity can lead to cancer of liver. Young men may suffer impotence, young women may suffer amenorrhea. Vitamin supplement for seniors have no iron, its not needed. Men should stop taking iron supplements at 20. women should stop at menopause. There is no simple visible symptom that will warn you before you wind up seeing a physician. Physician will find out from the blood test and not from the symptoms.
- Q: the doctor just rung and said i had to much iron. something to do with my liver?
- you'll become IRON WOMAN, lol just kidding, you'll get vitamin poison.
- Q: can iron oxide be reduced in molten form?please dont explain me how they do in blast furnace.its not what i am asking.simply if you have iron oxide powder and carbon powder which is from coal,if you mix them and melt in graphite crucible,does it reduce?no air blowing nothing.air free.melted with carbon powder.does it reduce?thank you
- The short answer is yes but the real answer is it depends. You are talking about melting iron oxide so which iron oxide do you have? There are 3 and the melting points are different. Then you need to look at the atmosphere, the stability of iron oxides as f(temp) depends on the oxygen, CO, CO2, hydrogen partial pressures (and the presence of any other reducing gases). Of course if you do reduce the oxide to iron metal, it will react with the graphite crucible to form Fe3C, iron carbide, or, depending on the atmosphere and temperature, maybe cast iron. Consult your friendly neighborhood extractive steel metallurgist.
- Q: I want to iron the tongue of my shoe because it has some weird curvature to it. Thus, I want to make it straight. I want to know if that is possible. THANX!!!!
- NOOOOOOOOOOOOOOOO!!!!!!!!!!
- Q: I am aware that naturally occurring rust is iron oxide and the iron involved is a Fe(III) with a plus 3 charge. How are you able to produce ferrous oxide or Fe(II)O with the iron having a plus 2 charge? Is the iron(II) a low abundance isotope of iron and where could you possibly find this in nature or how could you experimentally produce that specific iron?
- Naturally occurring rust is actually hydrated iron(III) oxide, either Fe(OH)3 in water or FeO(OH) in temperate air. Fe2O3 is actually dehydrated rust. The formation of rust requies not only oxygen, but water, as well. 4Fe(s) + 3O2(g) + 2H2O(l) -- 4FeO(OH)(s) Iron can form various iron(II) oxides on the way to FeO(OH), but they would only be intermediate products. The isotope of iron will have little to no impact on the ultimate oxidation product. Iron-56 makes up almost 92% of stable Fe atoms. All of the isotopes of iron have the same electron arrangement, and therefore, the same chemistry.
- Q: Ok. so I was reading this story a friend wrote about someone who committed suicide by overdosing on iron? Can this actually happen and how much would it take? It seems like the body would just get rid of the excess or something.
- It is a fact that you can overdose on water*, so I think it's very possible to overdose on anything. Lets not get into specifics of amounts, types, substance, or methods of ingestion. The human body is a marvelous organic machine that needs to be treated with wisdom. Everything in moderation. Their was a college sorority group that had a water drinking game instead of alcohol for initiation. Sounds safe . One contestant died after drinking (?)gallons of water in (?) amount of time. Google it
Send your message to us
ductile iron double flanged reducer
- Loading Port:
- China Main Port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords