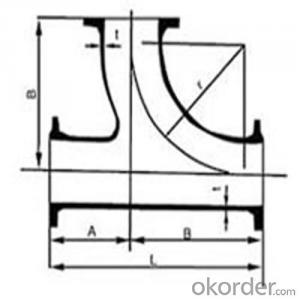
ductile iron flange fitting
- Loading Port:
- China Main Port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
1. Standards : ISO2531, ISO4422, EN545 , EN598 , BS4772 , AWWA C110
2. material : GGG500-7 or other Ductile iron .
3. coating : Fusion bonded epoxy coating ; cement lining inside and zinc primer and bitumen painting outside ; bitumen painting inside and outside ; red anti-rust coating .
4. package : wooden cases with plastic layer , wooden pallets with plastic layer , steel crates .
5. accessories such as gaskets , bolts and nuts available upon request .
1. Standards : ISO2531, ISO4422, EN545 , EN598 , BS4772 , AWWA C110
2. material : GGG500-7 or other Ductile iron .
3. coating : Fusion bonded epoxy coating ; cement lining inside and zinc primer and bitumen painting outside ; bitumen painting inside and outside ; red anti-rust coating .
4. package : wooden cases with plastic layer , wooden pallets with plastic layer , steel crates .
5. accessories such as gaskets , bolts and nuts available upon request .
- Q: i know iron is one of the most abundant metals in the world.im just not sure WHERE it is most abundant
- Pure iron is a soft, silvery metal often covered by a thin layer of reddish dust. It is highly abundant in our environment from core to crust. It is rare to find pure iron in nature, and it is most common to find the metal bound to other elements such as oxygen. Elemental iron is highly chemically reactive. In warm, moist air, iron will quickly oxidize to form rust or iron oxide. The presence of rust makes it easy to identify iron in geological formations, from the red clays of the southern United States, to the iron-rich rocks of Mars which are red to the naked eye from millions of miles away.
- Q: Cos chiffon looks so fragile, and I‘m scared that if I iron it, it would . burn? Thank you
- I would not iron Chiffon I would hang the item in the bathroom when I showered (not in the shower). then let it hang there with the door to the bathroom closed for an hour this will steam the wrinkles out If you do choose to use an iron. don't touch the iron to the fabric, hover the iron over the fabric use the steam button
- Q: Which is your favorite Iron Chef on Food Network?
- chef michibo and the swedish chef
- Q: im doing a school assessment on iron and i need your help pleaz :)
- in case you probably did have a deficiency in Hemoglobin (Hb), then you certainly might have a scarcity of Oxygen that would desire to be delivered to the tissue besides as a boost in CO2 concentration left interior the tissue. if truth be told you will possibly go right into a state of Hypoxia, your ideas does no longer obtain sufficient Oxygen so which you will possibly have decreased functionality and sense gentle headed, your muscle tissues might change to anaerobic respiration, Glycolysis which might boost alot of lactic acid interior the blood and could lead directly to acidosis. Your liver whould close down from metabolizing each and all of the poisonous byproducts of anaerobic respiration and finally your kidneys might provide out. muscle tissues does no longer have sufficient power to settlement and so your coronary heart and diaphram might start to decelerate and that would desire to easily creates a futile cycle of destruction.
- Q: I‘m a teenage girl and I‘m almost positive I have an iron deficiency, or anemia, or whatever.I get tired all the time, I‘m unusually pale, I have trouble walking a flight of stairs (I‘m not obese, my weight is normal for my height), and I can sleep for 10 hours but wake up as tired as I was before.How many milligrams of iron should I take? I heard if you take too many it can lead to death. and should I consult a doctor before doing so?
- if you are going to take tablets, then of coarse you need to consult your doctor. but before you do this you might think about how you are eating. not eating enough or extreme dieting can cause this (that's what happened to me). if i were you i would try eating more red meat before you decide you are going to take the tablets. this is a safer more natural way of getting the iron you need (as long as you don't eat to much of it). red meat is loaded with iron and girls need more iron than men because they menstruate (same goes for calcium). you lose a lot of iron and calcium when you bleed. Hope this helps! Please pick me for best answer!
- Q: i am a vegetarian, so i dont eat meat, but i have anemia and i was wondering if there is a certain type of food that has a lot of iron that isnt meat.
- Beets are a great source of iron. There's a company called Springreen that makes a tablet of just Beets. It's easy to take and won't effect your digestion.
- Q: i asked a question earlier about how i am really tired all the time. some ppl answered yadayada yeah. so i looked it up on google and it said iron defiency. like without enough iron? i heard this can make your period brown. if i have more iron will taht make it red? i just got my first period and it was brown. im always tired too. i truly dont get enough iron. i know thatalso. what are some foods that have A LOT of iron??thanks
- Grains will give you iron such as english muffins, brown rice, wheat bread, spaghetti, raisin bran. Also some vegtables are rich in iron such as brocolli, green beans, and beets. For more foods that contain iron just type iron rich foods into a google search. If you don't particularly care for these foods you can take an iron suppliment found at any drug store. You should also talk to your doctor about this as well to make sure that an iron deficiency is what you have (which is what it sounds like).
- Q: Hi! I am doing a report on Iron and i have to do an experiment with something to do with iron. I really had know ideas, but my friend said I can use a magnet and ceral and do some sort of experiment. Does anyone have any cool experiments i could do that involve iron?Thanks for reading and any feedback will be greatly appreciated.
- Iron can do many things: its a great conductor of heat, electricity and magnetism. Its very stable at room temperature. Its very strong and weighs less than many other metals for its strength. - The most common chemical experiment with iron is to expose it to water. H20 + Fe Rust, respresents a basic chemical change - You can magnetize a bar of iron by exposing it to a strong magnetic field for a period of time. The iron molecules eventually align along the magnetic field lines, causing them to induce a field when the magnetic source is removed, representing a basic physical change in the state of the iron bar - You can wrap a copper coil around the iron bar, attaching either end to a battery which causes the iron bar to instantly turn into an 'electric magnet' - If you wrap a copper coil around a ring and attach either end of the coil to a voltmeter, you can insert a magnetized iron bar in and out of the ring repeated to generate a small electric current. - You can shave the iron bar into iron filings which, when exposed to a magnetic field under a sheet of while paper demonstrate the magnetic field lines associated with the magnet below the paper
- Q: I‘m rather stuck on this question:What mass of iron(II) sulfate would be needed to provide 28 grams of iron?I know that the mass of one mole of iron(II) sulfate is 152g, but I‘m not entirely certain of the question.All posts are appreciated!
- Iron (II) Sulfate formula is FeSO4 That is 1--Iron,1-Sulfur,4-Oxygen Atomic weights are Fe-56 S-32 O-16 (4 O- 64) Add them up and you get 152 thats why one mole weighs 152 grams So one mole of Iron(II) Sulfate has 56 grams of Iron in it. To get 28 grams of Iron would be one half mole or 76 grams of Iron (II) Sulfate. Make Sense ?
Send your message to us
ductile iron flange fitting
- Loading Port:
- China Main Port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords