Amino Trimethylene Phosphonic Acid Water Treatment Chemical
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Product Description:
Amino tris(methylene phosphonic acid) / Amino Trimethylene Phosphonic Acid/ ATMP / 6419-19-8 / C3H12NO9P3
CAS No. 6419-19-8
Molecular Formula: N(CH2PO3H2)3
Molecular weight: 299.05
Structural Formula: 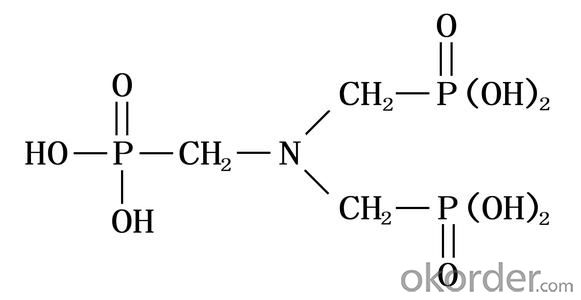
Properties:
ATMP has excellent chelation, low threshold inhibition and lattice distortion ability. It can prevent scale formation, calcium carbonate in particular, in water system. ATMP has good chemical stability and is hard to be hydrolyzed in water system. At high concentration, it has good corrosion inhibition.
ATMP is used in industrial circulating cool water system and oilfield water pipeline in fields of thermal power plant and oil refinery plant. ATMP can decrease scale formation and inhibit corrosion of metal equipment and pipeline. ATMP can be used as chelating agent in woven and dyeing industries and as metal surface treatment agent.
The solid state of ATMP is crystal powder, soluble in water, easily deliquescence, suitable for usage in winter and freezing districts. Because of its high purity, it can be used in woven & dyeing industries and as metal surface treatment agent.
Specification:
| Items | Index | |
|---|---|---|
| Standard | Solid | |
| Appearance | Clear, Colorless to pale yellow aqueous solution | White crystal powder |
| Active acid % | 50.0-51.0 | 95.0min |
| Chloride (as Cl-)% | 1.0 max | 1.0 max |
| pH value (1% solution) | 2.0 max | 2.0 max |
| Fe,mg/L | 10.0max | 20.0max |
| Density (20°C)g/cm3 | 1.31-1.35 | - |
| Colour APHA (Hazen) | 30.0max | - |
Application range&using method:
ATMP is usually used together with other organophosphoric acid, polycarboxylic acid and salt to built all organic alkaline water treatment agent. ATMP can be used in many different circulating cool water system. The recommended dosage is 5-20mg/L. As corrosion inhibitor, The recommended dosage is 20-80mg/L.
Package and Storage:
ATMP liquid: Normally In 30kg or 250kg net Plastic Drum;ATMP solid: 25kg inner liner polyethylene (PE) bag, outer plastic woven bag, or confirmed by clients request.Storage for ten months in room shady and dry place.
Safety Protection:
ATMP is Acidity, Avoid contact with eye and skin, once contacted, flush with water.
Shipping Date: Within 7-10 workdays after receiving your deposit.
Our Service:
Own Lab and joint venture factory.
Superb r&d team;Safety standardization production.
Rich experience in export and strong logistical support.
Good relationship with many large domestic pharmaceutical factory.
Perfect service, perfect supply chain.
- Q: Chemical production of iodine and magnesium with water as catalyst!
- In the 250mL three bottles were equipped with spherical condenser and constant pressure dropping funnel, in the condensate tube connected to the anhydrous calcium chloride drying tube. The flask was placed with 1.5 g of magnesium chip and a small tablet of iodine, 10 g of bromobenzene and 30 mL of anhydrous ether were mixed in a constant pressure dropping funnel. First 1/4 of the mixture into the flask, a few minutes later see the magnesium surface of the bubble generated, the solution was slightly cloudy, iodine color began to disappear. If no reaction occurs, use a hot water bath. After the start of the reaction, stir, slowly dropping the remaining bromophene ether solution, dropping the rate to keep the solution was slightly boiling state, after adding, in the water bath to continue reflow 0.5h, magnesium tablets full effect.
- Q: What is the principle of catalyst reaction rate in chemical reactions?
- Whether the chemical reaction can be carried out according to the change of free energy, but only according to the change of free energy can not determine whether the reaction can be completed, because the chemical reaction is also completed by the reaction of the energy barrier, that is, if the reaction energy barrier is high, To provide some energy, across the barrier, to complete the reaction. The energy barrier is called activation energy. And the role of the catalyst is to reduce the activation energy, so that in a relatively harsh environment, chemical reaction occurs.
- Q: What are the catalysts that appear in the chemistry experiment?
- Oxygen Oxygen Oxygen Oxygen also used when the catalyst is manganese dioxide MnO2
- Q: What is a Catalyst?
- Catalyst is a chemical substance which is used in chemical reactions in relatively small amounts to start up or increase the rate of the reaction without being consumed in the process. For example, Sulfuric Acid is used to dehydrate Ethanol to Ethylene. Enzymes in living beings are biological catalysts. Manganese dioxide, used to decompose Hydrogen Peroxide to Oxygen and Water. For detailed answer.... en.wikipedia.org/wiki/Catalysis
- Q: Horseradish enzyme catalyzed Luminol chemiluminescence reaction
- Disinfectant ah ~ bleach ah ~ ~ take this kind of thing to wash the blood once something can interfere with Lumino identification. So that want to do bad things must be a good plan. Lumino in the presence of copper, copper alloy, horseradish or some bleach in the presence of fluorescence. So if the scene of the crime was bleached
- Q: Thorough explanation pls.
- Catalyst are the substances which increase the rate of reaction. They do not get consumed in the reaction but they participate in intermediate reactions. The catalyst action can be explained as- Providing alternate energy path- Let us suppose that an endothermic reaction need 15 joules of threshold energy to occur. The catalyst will provide them path which needs less energy. Providing Surface- Many reaction may occur slowly because less contact between the molecules/atoms/ions or due to unavailability of proper structure, in this case the catalyst provide surface for carrying the reactions. There are several other actions which mayn't be necessary for you to understand the basic function of catalyst.
- Q: Will the catalyst be able to increase the rate of chemical reactions?
- Not necessarily, but junior high school, such as manganese dioxide is a positive catalyst, that is, "to speed up the reaction rate", when the teacher should also mentioned "to reduce the rate of response" situation
- Q: i keep messing up on those 2 simple things haha i would apprecaite some help.
- enzymes help biochemical reactions proceed at a faster rate than normal in a physiological system, catalysts or sometimes referred to as subunits, metals and other ligands, bind enzymes, and can have a positive and negative effect on the rate of a reaction. search them on wikipedia!
- Q: Now, i am studying for my biology exam in 3 weeks time...i stumbled upon catalase, and then checked my book its catalyst...now im confused...is there a different among these 2 terms? i think..catalyst is the when a substance brings up or about a chemical reaction without using itself up and then catalase breaks down the toxic by-product of metabolism, hydrogen peroxide, into water and oxygen.Or am i wrong?please explain what is catalyst and catalase in biology or are they the same, just differently?
- an enzyme that decomposes hydrogen peroxide into oxygen and water.a substance that causes or accelerates a chemical reaction without itself being affected. To put thing simply, a catalyst is any substance that speeds up a chemical reaction. These can be natural or manmade. Catalase is actually a specific type of naturally-occuring catalyst, an enzyme in cells that decomposes hydrogen peroxide (Which is extremely toxic to life!) into harmless components. Catalase enzymes are highly concentrated in the aptly named cell organelles known as peroxisomes. Just remember- if the word ends in -ase, it's a type of enzyme! :) Hope this information helps!
- Q: What is the analytical principle of chemical adsorbents? How about the number of active catalyst centers tested?
- What do you mean by the chemical adsorber? BET is the use of the surface of the uneven force field, but the inert gas at low temperature in the surface adsorption. TPD, TPR is the number of active centers that can be measured by the technique of desorption and reduction between specific gases and catalysts as the temperature increases. If the active site is a reduced position, H2-TPR can be used. If the active site is acidic, NH3-TPD can be used, but also the method of alkali titration.
Send your message to us
Amino Trimethylene Phosphonic Acid Water Treatment Chemical
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords






















