Amino Tri Methylene Phosphonic acid ATMP
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Product Description:
Amino tris(methylene phosphonic acid) / Amino Trimethylene Phosphonic Acid/ ATMP / 6419-19-8 / C3H12NO9P3
CAS No. 6419-19-8
Molecular Formula: N(CH2PO3H2)3
Molecular weight: 299.05
Structural Formula: 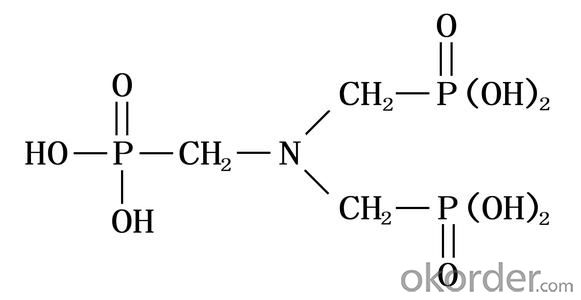
Properties:
ATMP has excellent chelation, low threshold inhibition and lattice distortion ability. It can prevent scale formation, calcium carbonate in particular, in water system. ATMP has good chemical stability and is hard to be hydrolyzed in water system. At high concentration, it has good corrosion inhibition.
ATMP is used in industrial circulating cool water system and oilfield water pipeline in fields of thermal power plant and oil refinery plant. ATMP can decrease scale formation and inhibit corrosion of metal equipment and pipeline. ATMP can be used as chelating agent in woven and dyeing industries and as metal surface treatment agent.
The solid state of ATMP is crystal powder, soluble in water, easily deliquescence, suitable for usage in winter and freezing districts. Because of its high purity, it can be used in woven & dyeing industries and as metal surface treatment agent.
Specification:
| Items | Index | |
|---|---|---|
| Standard | Solid | |
| Appearance | Clear, Colorless to pale yellow aqueous solution | White crystal powder |
| Active acid % | 50.0-51.0 | 95.0min |
| Chloride (as Cl-)% | 1.0 max | 1.0 max |
| pH value (1% solution) | 2.0 max | 2.0 max |
| Fe,mg/L | 10.0max | 20.0max |
| Density (20°C)g/cm3 | 1.31-1.35 | - |
| Colour APHA (Hazen) | 30.0max | - |
Application range&using method:
ATMP is usually used together with other organophosphoric acid, polycarboxylic acid and salt to built all organic alkaline water treatment agent. ATMP can be used in many different circulating cool water system. The recommended dosage is 5-20mg/L. As corrosion inhibitor, The recommended dosage is 20-80mg/L.
Package and Storage:
ATMP liquid: Normally In 30kg or 250kg net Plastic Drum;ATMP solid: 25kg inner liner polyethylene (PE) bag, outer plastic woven bag, or confirmed by clients request.Storage for ten months in room shady and dry place.
Safety Protection:
ATMP is Acidity, Avoid contact with eye and skin, once contacted, flush with water.
Shipping Date: Within 7-10 workdays after receiving your deposit.
Our Service:
Own Lab and joint venture factory.
Superb r&d team;Safety standardization production.
Rich experience in export and strong logistical support.
Good relationship with many large domestic pharmaceutical factory.
Perfect service, perfect supply chain.
- Q: The chemical equation of heating reaction of benzene and hydrogen under the action of catalyst
- C6H6 benzene + 3H2 - (arrow) C6H12 cyclohexane (Ni catalytic heating)
- Q: The "one-to-two change" of the catalyst is that the quality and chemical properties of the reactants are constant or the quality and chemical properties of the catalyst are constant?
- The quality and chemical properties of the catalyst are unchanged
- Q: What is a catalyst?
- The catalyst can change the reaction rate (either fast or slow), but the catalyst itself is not affected before and after the reaction, that is, the quality of the same, the chemical nature of the same, itself has not changed.
- Q: Could you please explain it, i know they increase reaction rates but how?
- A catalyst provides an alternative route for the reaction, (maybe more steps than previously), but each step having a lower activation energy than the original uncatalysed reaction. This means that although there will be the same number of collisions per second (if the reaction is performed at the same temperature as before), a greater fraction of those collisions will result in a reaction - so there will be more reactions per second. In the case of a heterogeneous catalyst - e.g. a solid surface the change is that the first step is a bond to the surface which waekens some of the bonds in the reactants - again making a greater fraction of reactions result in reaction.
- Q: Who knows hydrogen and nitrogen in the high temperature, high pressure and catalyst conditions for the synthesis of ammonia chemical equation ah? Urgent! The SOS
- 3H2 + N2 ===== 2NH3
- Q: In the catalyst and light conditions to break down the water to get the chemical equation of hydrogen
- 2H2O = (light or catalyst) 2H2 ↑ + O2 ↑
- Q: What is the difference between a catalyst and an oxidizing agent?
- A catalyst alters the rate of a reaction without being used up in the reaction. An oxidising agent oxidises other compounds, the agent itself being reduced in the process.
- Q: Hydrogen and nitrogen in the high temperature and pressure and catalyst conditions for the synthesis of ammonia chemical equation
- 3H2 + N2 catalyst iron ---> 2NH3 conditions high temperature and high pressure
- Q: Before and after the chemical reaction, the nature of the catalyst unchanged this statement right? Why?
- Only catalyze, do not participate in the reaction! The
- Q: Is it not the rate to accelerate the addition of the catalyst to the catalyst, and that is why the balance does not move
- In the chemical equilibrium, after adding the catalyst, the positive and negative reaction rate increases equally, but the positive reaction rate is still equal to the reverse reaction rate, so the balance does not move
Send your message to us
Amino Tri Methylene Phosphonic acid ATMP
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches





















