High-Quality Aluminum Foils Air Conditioner Insulation Hose
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 50000 pc
- Supply Capability:
- 100000 pc/month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Specification
1.Structure of Air Conditioner Insulation Hose Description:
Air Conditioner Insulation Hose is made out of aluminum and can be bent to allow for curves and offsets in the duct run. While it is best to use rigid duct from the dryer to the wall termination, clearance issues may require the use of semi-rigid duct. Semi-rigid duct may also be used as a temporary connection until you can install rigid duct. It is a much better choice than flexible ducting as it will not collapse on itself and the inside is smoother which prevents trapping and buildup of lint.
2.Main Features of the Air Conditioner Insulation Hose:
1). Aluminum
2). Hvac/ventilation
3). Heat resistance
3. Air Conditioner Insulation Hose Images


4. Air Conditioner Insulation Hose Specification:
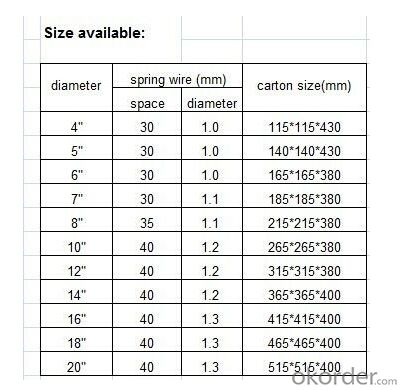
5.FAQ
1)How about our company?
We are a professional manaufactory
2)How about your quality?
We have a professional quality control team and we can guarrantee the quality.
3)Can you provide sample?
Yes, We can .
- Q: I've never had a toaster oven, and but my friend gave me one this weekIt's reeeally niceI've cooked fish in it, but how would I go about cooking a steak? I have a marinated/with garlic/onion/herbs steak and i'm not certain if I should put it in foil or what? how long should I could it and at what degreesIt's about a half of inch thick.I'm going to cook it tonight
- It doesn't matter what side touches the foodIf you really want to keep things hot, may I recommend investing in a thermos? The downside is the kids remembering to bring them homeThe only disadvantage I am aware of is there have been some links between aluminium and Alzheimer's diseaseI haven't seen anything conclusive though.
- Q: i want to polish a aluminum tube to make it shinewhat materials would i need?
- Don't put foil in the MW or you can burn your house downThe oven is a good alternativePlace your chicken in a pan, cover with foil to seal in the juices and keep your meat moist, then cook 20 minutes at 425F for boneless or 45 minutes for bone-in.
- Q: How do you convert 1.25 x 15^5 mols Al(NO3)3 to grams? please help?
- Hi! Unfortunately there is no way to transfer over long distances through bluetoothAlternative ways can be to transfer the image through bluetooth to a computer and email from thereAlmost all other ways require you to have a usb cable or network coverage in order to do this.
- Q: iron, magnesium, sulfur, lithium, zinc, iodine, oxygen, barium, aluminum, hydrogen, xenon, and copper?
- Look in the periodic tableeach group number on top represents the number of valence electrons For Example: Magnesium is in group 2 it has two valence electrons, Oxygen is in group 16 and it has 6 valence electrons.
- Q: How do i spray my BMX and also how can i spray DECALS to it????How do i spray my bmx step by step?? ( Like add primer than spray etc etc) Also , how do put the layers (Even/and all).And how do i add decals to it? Like you know print it on a paper and spray
- Hello JonathonYou don't need insulated blinds for windowsYou are covering glass,because glass loses heat.But wooden blinds are not much betterAll the rage at moment,but get dusty and look heavy in some areasSo long as you cover windows you can use-Roller,Roman, or special blindsHave you tried Martha Stewart site-type in blinds? There is a neat blind on sale here in New Zealand,which is transparent,but dense enough to keep heat inIt's great in areas which have bright sunLets light in-keeps glare outGood Luck!
- Q: I'm just wondering with the holidays coming up and allI'm not asking about reusing (which I will do with whatever paper possible) - I am talking about recycling itThanks!
- no it should be fine my parents do it all the time ^_^ good luck ! ^_^
- Q: I saw this once on MasterChef and one of the contestant used a frying pan covered with foil on top then sealed with the lid in place of an ovenI want to practice this but it is actually safe to do?
- Here are the main targets and goals to losing weight - Eat right: NO JUNK FOOD OR SODA WHATSOEVERTHIS IS THE MOST IMPORTANT AND HARDEST! - Exercise: You need to exercise at least 1 hour a day to burn off the calories you ate to see results - Weigh yourself: Don't weigh yourself randomlyWeigh yourself at a specific day and time every weekFor example: You could weigh yourself at Friday at 8:00am BUT KEEP IT THE SAME EACH WEEK or your weight will mess up and it won't be correct - Drink - Yes, drink fluids! Drink healthy fluidsDon't have too much GatoradeGatorade is around 80 calories in each bottle and it just adds up unnecessary calories when you could be having 0 calories of water instead - Rest: Sleep for at least 8 hours a day so you are well rested- Don't obsess: Don't obsess! Don't go too crazy about thisEat around 1,200 calories a day NO MATTER WHATIf you don't your body will go into a thing called starvation mode and it will mess up your metabolismSome good exercises: Jump rope, swimming, elliptical, walking the dog, stair climbing, pilates, crunches, push-ups, etcFood: STAY AWAY FROM JUNK FOOD AND SODA (as stated above)Replace cravings of junk food with vegetables and/or fruitsFruits and veggies have little calories but can fill you up for a few hours! THE LINK I WILL PROVIDE BELOW FOR YOU IS A SITE I USEIt isn't one of those lose weight sites, but it is a site where you can keep track of what you eat and how many calories are in itYou can also put in the exercises you did and it will tell you how many calories you burnIt also gives you recipes and tips to excessive weight loss!
- Q: Then it got caught on fire (because of the aluminum foil in the food's box)I have extinguished the fire with some bowls of tap waterMy question is, what would happen with the microwave? Will it be broken after what had happened?
- Recycling aluminum is so efficient that even Penn and Teller think it's a good idea - and they're very opposed to recycling Yes, recycling metals has always been done, long before there was ever an environmental reason to recycleIt's just cheaper to re-melt metals than make new metal from ore That is most true for aluminum, making new aluminum out of ore takes really staggering amounts of energy.
- Q: do i just stick foil around the edges of the pie crust i need a picture of this or something lol help please
- All those old stories about aluminium being unhealthy for cooking are just nonsense.Why would they be allowed to sell them if they were harmful? I have even washed re-used them at times.
- Q: Ok so I need I make the horns, I'm cosplaying a trollEveryone told me to get air dry clay, so I got basic crayola air dry clay and made some hornsBut these were super heavy! I couldn't even look up or down because they would fall off! What kind of clay should I get that's cheap and feather light?
- it is a poor insulator
Send your message to us
High-Quality Aluminum Foils Air Conditioner Insulation Hose
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 50000 pc
- Supply Capability:
- 100000 pc/month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords


























