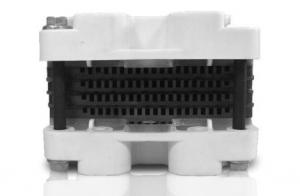
Fuel Cell Stack for Portable Power
- Loading Port:
- China Main Port
- Payment Terms:
- TT or LC
- Min Order Qty:
- 1 Unit unit
- Supply Capability:
- 5000 Units Per Year unit/month
OKorder Service Pledge
Quality Product, Order Online Tracking, Timely Delivery
OKorder Financial Service
Credit Rating, Credit Services, Credit Purchasing
You Might Also Like
EOS0403 fuel cell is a highly integrated fuel cell system with compact design.5W is a suitable power for the mobile applications.
Products characters:
1. Light weight and small size;
2. Low noise;
3. Simple system and high reliability;
4. Quick startup, good dynamic performance;
5. Excellent environment adaptability;
6. Simple control and communication policy
- Q: Does anyone know why batteries have successfully been commercialized where as fuel cells (solid oxide ones in particular) have not? I'm looking for articles regarding the subject! Thanks,N
- Batteries have been successfully commercialized because they are cheap to make. Fuel cells aren't yet so they haven't. Once they are affordable they will be much more common.
- Q: I know fuel cells are non-renewable, but why are they non-renewable?
- Because they run out of .fuel?
- Q: i dont like the smaller fuel cells that nascar uses at some tracks. i go to and watch races to watch the cars race each other, not to see the race turn into the fastest pit stops, i want to see more racing on the track, also if nascar is so worried about the people that race, or pit WHY HAVE MORE PIT STOPS, someday some pit crew member is going to get hurt bad or maybe God forbid something worst, because they had to make 2 or 3 more pit stops than they would have had they a bigger fuel cell, what will nascar do then, ok on lap 40 all the even number cars will pit, and on lap 41 all the odd number cars will pit, Will they say sorry we didnt think that would happen., dont think so
- The smaller fuel cell affects pits stops only during long green flag runs. Due to the varying fuel mileage and pit strategies this isn't a real problem because the green flag stops are staggered over about 3-5 laps. The problem you are describing happens during yellow flag pit stops when they all come in at the same time causing the dangerous situations. The smaller fuel cell doesn't matter at that time. They are all going to come in and make adjustments anyway so they are going to top off the tank regardless of the size of the fuel cell.
- Q: hydrogen is the most abundant element in the universe - we could never run out - it's what powers our sun, ergo, the entire solar system - the byproduct, I am told, is water - it seems to make much more sense than hybrid or electric - what is the problem?
- Come up with hydrogen fuel-cell solutions these days.
- Q: Hello. I have to make an essay about this topic for tomorrow. I have tried to find the answer on the internet but it's far too complicated for me. I'm 13. Could anyone please explain it to me in a simply? I'm kind of stressing out a little. I'll give 5 stars to the best answer. Oh, and if you could also please define the main characteristics o each one. Thank you very much!
- Fuel cells are used in hydrogen cars and hybrids run of of two energy sources. Like Gas and Electricity.
- Q: A little smaller or larger could have took fuel equation out of race. Everyone would have pushed harder instead of conserving gas the whole race. I mean it started on lap one. Sorry for all the whining but I am one of the few here who enjoy watching the road races. Damn I just wanted to see the go all out
- yes not because of the new fuel cell but because it and the COT made it impossible to really get a feel for whether the teams could eliminate a stop. Normally it is no big deal on an oval since pitting means losing a lap, pitting under the yellow is almost a given but on a road course where you almost never want to pit under yellow it was huge
- Q: Assuming that the technology is implemented, what would be the consequences?
- Interesting question, after a brief study some time ago here is my understanding: Hydrogen stored in liquid form is used to run the fuel cells. Normal hydrogen gas is about 75% ortho (parallel spins) and 25% para antiparallel spins. The conversion of hydrogen gas to a cryogenic liquid is highly inefficient: The following heats must be removed: specific, latent, and latent ortho-para. The amount of energy used to convert hydrogen gas to the liquid state with reference to the ortho-para conversion alone is about: 6,000,000 J/kg since in the liquid state, the product is nearly 100% para-hydrogen. To further complicate matters this phase of the conversion can take days to complete depending on the specific process. Further energy losses due to storage, delivery, safty issues, etc. result in more overall inefficiency. Currently, I don't know if these issues have been resolved. Put simply, a great deal of energy must used just to fill the darn fuel cell tank. Hence, there may be environmental consequences as a result of the production process while fuel cell operation itself may seem harmless. I think the argument of increased greenhouse water vapor in the atmosphere as a result of global usage of fuel cells doesn't hold water. I'll omit my counter argument. Earth physics is a very complicated matter. I have no idea what would really happen. Many times nature has a way of surprising us despite all our computer models and seemingly plausible theories. More careful study is needed. Take sides if you must that's what lawyers do, but don't be surprised if you get overruled by nature.
- Q: I need this for my science class, I'm really tired and I want to be done with it. Please answer quick :-)
- A fuel cell is, very generally speaking, an enclosed space capable of producing energy which can be harvested for work. The most practical example of a fuel-cell is a battery. Fuel-cells work by separating charged molecules and just creating an electric potential (i.e. voltage). You can make very rudimentary fuel-cells in a lab using two beakers filled with particular solutions (which produce opposing charges), a conducting wire such as copper or platinum which connects the separated beakers, and a salt bridge, so that equalization of charge can occur and the fuel-cell can be used over and over again. In a special case, the biotic cell can be thought of as a fuel-cell as the plasma membrane has a voltage across it due to the unequal distribution of charged ions on either side of it (either outside the cell or inside the cell). I can give you a much more detailed answer if you like, but have you taken general physics or chemistry yet?
- Q: has anyone tried the systems on thier own car . pros n .
- They have been running some buses in CA on fuel-cells. It has cost them 75% more to do so than the standed bus.
- Q: i was wondering what the purpose is?does it help save gas?
- A fuel cell is what holds the fuel it self, like a gas tank. Usually fuel cells are used in many types of auto racing.
To impact our industry, our society and our planet with our greatest effort to develop green technology, PEARL commits efforts to being a low-cost solution expert in the hydrogen energy industry. PEARL hopes to cooperate with men of insight to promote the development of hydrogen energy economy and to bring you a zero-emission environment and a bright future.
1. Manufacturer Overview
| Location | Shanghai,China (Mainland) |
| Year Established | 2006 |
| Annual Output Value | US$1 Million - US$2.5 Million |
| Main Markets | North America; Southeast Asia; Western Europe |
| Company Certifications | CE Certificate; ISO9001:2008 |
2. Manufacturer Certificates
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period |
3. Manufacturer Capability
| a) Trade Capacity | |
| Nearest Port | |
| Export Percentage | 61% - 70% |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b) Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | OEM Service Offered Design Service Offered |
| Product Price Range | |
Send your message to us
Fuel Cell Stack for Portable Power
- Loading Port:
- China Main Port
- Payment Terms:
- TT or LC
- Min Order Qty:
- 1 Unit unit
- Supply Capability:
- 5000 Units Per Year unit/month
OKorder Service Pledge
Quality Product, Order Online Tracking, Timely Delivery
OKorder Financial Service
Credit Rating, Credit Services, Credit Purchasing
Similar products
Hot products
Hot Searches
Related keywords