Water Treatment Solid Amino Trimethylene Phosphonic Acid
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Product Description:
Amino tris(methylene phosphonic acid) / Amino Trimethylene Phosphonic Acid/ ATMP / 6419-19-8 / C3H12NO9P3
CAS No. 6419-19-8
Molecular Formula: N(CH2PO3H2)3
Molecular weight: 299.05
Structural Formula: 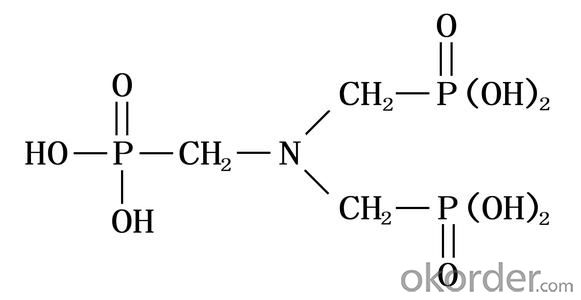
Properties:
ATMP has excellent chelation, low threshold inhibition and lattice distortion ability. It can prevent scale formation, calcium carbonate in particular, in water system. ATMP has good chemical stability and is hard to be hydrolyzed in water system. At high concentration, it has good corrosion inhibition.
ATMP is used in industrial circulating cool water system and oilfield water pipeline in fields of thermal power plant and oil refinery plant. ATMP can decrease scale formation and inhibit corrosion of metal equipment and pipeline. ATMP can be used as chelating agent in woven and dyeing industries and as metal surface treatment agent.
The solid state of ATMP is crystal powder, soluble in water, easily deliquescence, suitable for usage in winter and freezing districts. Because of its high purity, it can be used in woven & dyeing industries and as metal surface treatment agent.
Specification:
| Items | Index | |
|---|---|---|
| Standard | Solid | |
| Appearance | Clear, Colorless to pale yellow aqueous solution | White crystal powder |
| Active acid % | 50.0-51.0 | 95.0min |
| Chloride (as Cl-)% | 1.0 max | 1.0 max |
| pH value (1% solution) | 2.0 max | 2.0 max |
| Fe,mg/L | 10.0max | 20.0max |
| Density (20°C)g/cm3 | 1.31-1.35 | - |
| Colour APHA (Hazen) | 30.0max | - |
Application range&using method:
ATMP is usually used together with other organophosphoric acid, polycarboxylic acid and salt to built all organic alkaline water treatment agent. ATMP can be used in many different circulating cool water system. The recommended dosage is 5-20mg/L. As corrosion inhibitor, The recommended dosage is 20-80mg/L.
Package and Storage:
ATMP liquid: Normally In 30kg or 250kg net Plastic Drum;ATMP solid: 25kg inner liner polyethylene (PE) bag, outer plastic woven bag, or confirmed by clients request.Storage for ten months in room shady and dry place.
Safety Protection:
ATMP is Acidity, Avoid contact with eye and skin, once contacted, flush with water.
Shipping Date: Within 7-10 workdays after receiving your deposit.
Our Service:
Own Lab and joint venture factory.
Superb r&d team;Safety standardization production.
Rich experience in export and strong logistical support.
Good relationship with many large domestic pharmaceutical factory.
Perfect service, perfect supply chain.
- Q: What is the chemical vinyl chloride and benzene plus catalyst?
- Substituting the reaction to produce styrene and removing HCl
- Q: What is the maximum impact of the chemical reaction rate? Such as catalyst, temperature, concentration. If you can, you can row order.
- The catalyst certainly affects the most
- Q: Chemistry why the catalyst can be equal to speed up the positive reaction rate
- Since the catalyst only changes the activation energy and the amount of reactivity can be reduced, the amount of reaction energy is also reduced. Therefore, a positive catalyst is also a good catalyst for its reaction. Speed up the same multiple.
- Q: What is the microcosmic principle of the catalytic reaction in the chemical reaction?
- The catalyst reduces the activation rate of the reactants by increasing the reactant density of the reaction conditions and making the chemical reaction easier.
- Q: Comparison of biocatalysts with chemical catalysts!
- (1) The chemical reaction catalyzed by the biological enzyme is generally carried out under relatively mild conditions. (2) The enzyme has the highest activity at the optimum temperature and pH, and the temperature of the biocatalyst is more moderate. And PH high or low, the enzyme activity will be significantly reduced.In general, the animal in the enzyme the optimal temperature between 35 ~ 40 ℃; plant enzyme in the optimal temperature between 40 ~ 50 ℃; animal body Of the enzyme most of the most suitable pH between 6.8.0, but there are exceptions, such as the optimal pH of pepsin 1.5; plant enzymes in the most suitable pH between 4.6.5. (3) acid, Or the temperature is too high, the enzyme structure will be destroyed, so that the enzyme permanently inactivated .0 ℃ or so, the enzyme activity is very low, but the spatial structure of the enzyme is stable, at the appropriate temperature of the enzyme activity can be increased The
- Q: Is it possible for the different chemical reactions to have the same catalyst?
- Right, think about the catalysis of biological enzymes
- Q: okay im doing a project for my classroom about catalyst and i have to draw a picture but when i looked up on google i just saw a bunch of random stuff and a couple were metal so thats why im asking this question. :)
- A catalyst most often is a metal in the form of a screen or sponge with lots of area although it can be an immiscible liquid or sand like particles that can be filtered out.
- Q: Describe the role of a catalyst and a substrate in a chemical reaction.
- Describe Catalysts
- Q: What is the relationship between the catalyst and the chemical reaction? What is the relationship between the enzyme and the catalyst?
- The catalyst can change the activation energy of the chemical reaction, thereby changing the reaction rate.
- Q: I know that a species that does not appear in the chemical equation may also affect the rate of a reaction - e.g. a catalyst. But does that mean the catalyst can be present in the rate equation, and if so are catalysts always present in the rate equation?
- Any reaction with a finite amount of reactants has a half-life, whether it's first order, second order, zero order or complex order. The half-life (t?) is defined as the time taken for the reaction to go half-way to completion. If the reaction is: A + B ---products and A is in excess, then t? will be the time taken for half of B to be used up. For all reactions, then, you get a decay curve. For zero-order reactions, this 'curve' is a straight line, but for all other orders, the curve is an actual curve and it is quite difficult to distinguish, by visual inspection alone, whether it is exponential (indicating a first-order reaction) or hyperbolic (indicating a second or higher order reaction).
Send your message to us
Water Treatment Solid Amino Trimethylene Phosphonic Acid
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches





















