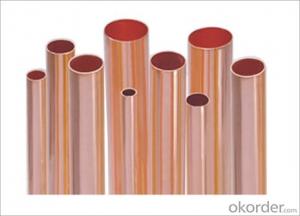
Refrigeration Copper Pipe for Refrigeration Devices
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 500 kg
- Supply Capability:
- 10000 kg/month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Name:Refrigeration Copper Pipe for Refrigerating
| Model: | P2、TU1、TU2、T2 |
| Brief: | Mainly used in refrigerating system of air conditioners; high precision and pressure-resistant product manufactured through extrusion, rolling, drawing, surface treatment, and heat treatment, usually processed into finned refrigeration pipe and other refrigeration devices. |
Type Specificationss:
Item | air conditioner copper pipe |
Type | straight copper pipe,pancake coil copper pipe,capillary copper pipe |
Standard | GB/T1527-2006,JIS H3300-2006,ASTM B75M,ASTMB42,ASTMB111,ASTMB395, ASTM B359,ASTM B188,ASTM B698,ASTM B640,etc |
Material | C10100,C10200,C10300,C10400,C10500,C10700,C10800,C10910,C10920, C10930,C11000,C11300,C11400,C11500,C11600,C12000,C12200,C12300, C12500,C14200,C14420,C14500,C14510,C14520,C14530,C17200,C19200, C21000,C23000,C26000,C27000,C27400,C28000,C33000,C33200,C37000, C44300,C44400,C44500,C60800,C63020,C65500,C68700,C70400,C70620, C71000,C71500,C71520,C71640,C72200,etc |
Shape | Round,Square,Rectangular,Oval,half-round |
Outside diameter | 4.76-28.58mm |
Wall thickness | 0.4-1.5mm |
Length | 1m,2m,3m,6m,or as required |
Hardness | 1/16 hard,1/8 hard,3/8 hard,1/4 hard,1/2hard,full hard,soft,. |
Surface | mill,polished,bright,oiled,hair line,brush,mirror,sand blast,or as required |
Price Term | Ex-Work,FOB,CNF,CFR,CIF etc |
Payment Terms | TT,L/C etc |
Export to | Singapore,Indonesia,Ukraine,Korea,Thailand,Viet Nam,Saudi Arabia,Brazil,Spain,Canada, USA,Egypt,Iran,India,Kuwait,Dubai,Oman,Kuwait,Peru,Mexico,Iraq,Russia,Malaysia,etc |
MOQ | 2 tons |
Package | Standard export package ,or as required. |
Application | Copper pipe have strong, corrosion resistant properties, and become a modern contractor in all of commodity house pipes, heating, cooling water piping installation of choice |
Contact | If you have any question ,please feel free to contact me we are sure your inquiry or requirements will get prompt attention |
Usage: Mainly used in refrigerating system of air conditioners; high precision and pressure-resistant product manufactured through extrusion, rolling, drawing, surface treatment, and heat treatment, usually processed into finned refrigeration pipe and other refrigeration devices.
Production Equipment
750-1500kg main frequency induction copper melting furnace, 80MN water seal extruder, LG60 high speed two-roll cold pilger mill, straight-line wire-drawing machine, copper tube/pipe straightener, polisher, 84′ copper coil winding machine, straightening-cutting & pancake coiling machine, thermoplastic packaging machine, 1.2T continuous bright annealing furnace.

80MN extruder

Continuous bright annealing furnace
Testing Devices
spectrum analyzer, atomic absorption analyzer, spectrophotometry, analytical balances, metallurgical microscope, eddy current flaw detector, metal tensile testing machine, eddy conductivity instrument.

Hardness tester

Spectrum analyzer

Metallurgical microscope

Metal tensile testing machine

Atomic absorption analyzer
Process
refined copper — smelting & casting — extrusion — rolling — drawing — straightening-cutting/forming of pancake coil — bright annealing — packaging — finished goods.
Standards
GB/T 1527-2006 Drawn tube of copper and copper alloys
GB/T 16866-2006 Dimensions and tolerances of copper and copper alloy seamless tubes
GB/T 4423-2007 Copper and copper-alloy cold-drawn rod and bar
GB/T 5231-2001 Wrought copper and copper alloys chemical composition limits and forms of wrought products
GB/T 5585.1-2005 Copper or aluminium and its alloy bus bars for electrical purposes—Part 1:Copper andcopper alloy bus bars
GB/T 17791-2007 Seamless copper tube for air conditioner and refrigeration equipment
GB/T 19850-2005 The seamless round copper tubes for electrical purposes
GB/T 26024-2010 Seamless copper and copper alloys tube for valves on air-conditioning and refrigeration system
ASTM B280-2008 Standard Specification for Seamless Copper Tube for Air Conditioning and Refrigeration Field Service
EN 12735-1:2001 Copper and copper alloys Seamless,round copper tubes for air-conditioning and refrigeration Part 1:Tubes for piping systems
EN 12735-2:2001 Copper and copper alloy – seamless round copper tube/pipe for air-conditioning and refrigeration system – for equipment
JIS H3300-2009 Seamless copper and copper alloy tube/pipe
- Q: A copper wire at room temperature that is 1 mm thick and 30 cm long is connected to a 1 V battery. If all of the power goes into heating up the wire, find the temperature of the wire in 10s. (the density of copper is 8.9 g/cm3 and specific heat is 0.39 J/g·°C) I can't figure out this answer correctly. Thanks!!
- the respond relies upon on a brilliant variety of factors. textile and length and thickness of the twine, and length and capability of the battery. If the twine gets too warm, it may react with the air. case in point, copper twine will turn green with copper oxide. Too warm and it will soften or set fireplace to this is environment. biking it on and stale should not be a difficulty, as long because it does not get too warm. Haw straight away it heats up and cools down (and that they are the comparable) relies upon, returned on the mass of the twine, the textile, and what this is touching. you will could test, start up wit a skinny twine, like #30, and a protracted length, possibly might up in a coil (you will could use magnet twine which has a layer of varnish on it) only as an occasion, in case you had a 9v battery and you wanted 3 watts of warmth, use P = E?/R 3 = 9?/R R = 27 ohms now use the resistivity formula, to calculate the dimensions Resistance of a twine in ? R = ?L/A ? is resistivity of the textile in ?-m L is length in meters A is circulate-sectional area in m? A = ?r?, r is radius of twine in m resistivity Cu 17.2e-9 ?-m #30 twine has a diameter of 0.25 mm or a radius of 0.000125 m it quite is a circulate-sectional area of A = ?r? = A = ?(0.000125)? = 4.9e-8 m? plugging into the resistivity formula 27 = (17.2e-9)L/(4.9e-8) L = 77 meters. this could be too long, so this is quite useful to objective for a thinner twine. Or swap to a twine with an more suitable resistivity. Nichrome, case in point, has a cost resistivity Nichrome 150e-8 ?-m 100x larger than copper. .
- Q: If you touch a test lead to a copper pipe in the house, and put the other test lead in a neutral outlet we get approx. 116 volts. An electrician came by and looked for reverse polarity in the outlets but nothing. Since the house was built in the 1930‘s we did have open grounds on the outlets which are fixed. The electrician seemed to think there could be a box buried somewhere and the wrong wires are tied together can anyone think of a fix on this since we can‘t find the box ? or a different check ? fix ?
- If you have an electric water heater turn off the breaker or pull the fuse to it and recheck the problem. You may have an element going bad in the water heater. If this does not help then start shutting down individual circuits until you locate which circuit is causing the problem and start backtracking from there.
- Q: was wondering how you did it, they are going to do it to my pipes that are connected to my washer but was wondering how it was done
- you clean the ends of the pipes that you are going to sweat together oh heck look at these two sights both give you videos and will give you a little info
- Q: Bathroom decoration when the dark pipe with copper or PP hose that kind of good?
- Copper tube, of course. It's expensive. Now PPR pipe is used
- Q: I am talking about unthreaded fittings.
- Eat the snow. Come on, be the man ! I know you have the balls to do it. I'm right with ya.
- Q: I‘m writing up my A2 physics coursework and i understand the concept of eddy currents and that it‘s because copper is a better conductor but i good explanation of how a better conductor leads to stronger eddy currents would be highly appricieated.Thankyou so much in advance !! :D
- Aluminum is a good conductor of electricity, but copper is better. A moving magnetic field generates an electric current and conversely an electric current creates a magnetic field (think electromagnet) Like magnetic poles repel and unlike fields attract. So.The falling magnet creates current which creates a counter magnetic force which affectgs the falling magnet. If you would build this in a circular pattern, and make the first magnet an electromagnet, then power it up you would have made an electric motor.
- Q: What are the costs per linear foot to remove asbestos covering copper heat pipe or to encapsulate it?
- Encapsulation will be more than removal these days. The costs for removal will vary by location of the facility and the location within the facility. For exposed pipe, without complications (kids, people, hot pipes), $1-2 per foot plus a flat setup fee is common. The only way to know for your location is to just get three bids from licensed contractors (they are free).
- Q: I have a copper canope above my fire but the copper has blue/green streaks. Standard brass/copper cleaner ( Brasso ) doesn't shift it. Any ideas ?
- I okorder
- Q: Had copper Iud inserted 2 days ago.. The pain at time of insertion was aweful, and 12 hours after.. felt like i was in labour.Now still having occasional sharp shooting pain in vagina and more so in my bum ???? Is this normal?? Another note.. I went back in yesterday cuz it still hurt and she checked on it, an dcouldnt find the strings.. so she doesnt think it was expelled, so i have to go for an ultrasound, but they r booking into late october.. any thought????
- My wife thinks It is possably the effects of Cervadil or any other cervial diolating medicine. Give it a couple more days and the cramping should stop. Or possably an allergic to the copper of the iud. I dont know if GOD intended for women to have copper in thier vaginas. We have concidered similar family planning options but opted not to because of these exact concerns. Good Luck and please let us know how it goes.
Send your message to us
Refrigeration Copper Pipe for Refrigeration Devices
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 500 kg
- Supply Capability:
- 10000 kg/month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords