Low Sulfur Calcined Petroleum Coke of CNBM in China
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 1 m.t.
- Supply Capability:
- 10000000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
1.Structure of Calcined Petroleum Coke Description
Calcined Petroleum Coke is made from raw petroleum coke,which is calcined in furnace at a high temperature(1200-1300℃).CPC/Calcined Petroleum Coke is widely used in steelmaking,castings manufacture and other metallurgical industry as a kind of recarburizer because of its high fixed carbon content,low sulfur content and high absorb rate.Besides,it is also a best kind of raw materials for producing artifical graphite(GPC/Graphitized Petroleum Coke) under the graphitizing temperature(2800℃).
2.Main Features of the Calcined Petroleum Coke
High-purity graphitized petroleum coke is made from high quality petroleum coke under a temperature of 2,500-3,500°C. As a high-purity carbon material, it has characteristics of high fixed carbon content, low sulfur, low ash, low porosity etc.It can be used as carbon raiser (Recarburizer) to produce high quality steel,cast iron and alloy.It can also be used in plastic and rubber as an additive.
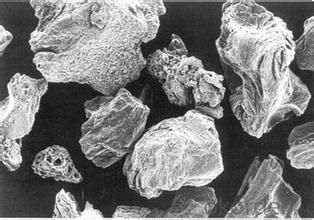
3. Calcined Petroleum Coke Images


4. Calcined Petroleum Coke Specification
| Place of Origin: | Jiangsu, China (Mainland) | Type: | Petroleum Coke | Calory (J): | 7783 |
| Sulphur Content (%): | 4 | Ash Content (%): | 0.5 | Fixed Carbon (%): | 86 |
| Moisture (%): | 7.3 | Volatile Matter (%): | 10.12 | Abrasive Resistance: | 70 |
| Size: | Different sizes |
5.FAQ of Calcined Petroleum Coke
1). Q: Are you a factory or trading company?
A: We are a factory.
2). Q: Where is your factory located? How can I visit there?
A: Our factory is located in ShanXi, HeNan, China. You are warmly welcomed to visit us!
3). Q: How can I get some samples?
A: Please connect me for samples
4). Q: Can the price be cheaper?
A: Of course, you will be offered a good discount for big amount.
- Q: How does carbon impact the stability of desert ecosystems?
- Desert ecosystems can be influenced both positively and negatively by carbon. On the positive side, carbon is crucial for all living organisms and is a vital component of organic matter. It plays a critical role in essential processes like photosynthesis, respiration, and decomposition that are necessary for the survival and growth of plants and other organisms in deserts. During photosynthesis, plants take in carbon dioxide, a type of carbon, to produce glucose and oxygen, which are essential for their growth. This supports the stability of desert ecosystems by promoting primary productivity and the food web. However, the excessive release of carbon into the atmosphere, primarily caused by human activities such as burning fossil fuels and deforestation, has resulted in an increase in greenhouse gases, including carbon dioxide. This leads to global warming and climate change, which have detrimental effects on desert ecosystems. The rising temperatures can disrupt the delicate balance of desert ecosystems, impacting the distribution and abundance of plant and animal species. Some plants may struggle to adapt to the changing climate while others may benefit, resulting in changes to species composition and the potential loss of biodiversity. Additionally, elevated levels of carbon dioxide can impact water availability in desert ecosystems. Higher carbon dioxide levels can enhance water-use efficiency in plants, allowing them to conserve water. While this can be advantageous in water-limited environments such as deserts, it can also alter water dynamics, affecting the availability of water resources for other organisms in the ecosystem. To summarize, carbon is essential for the stability of desert ecosystems as it supports primary productivity and the functioning of food webs. However, the excessive release of carbon into the atmosphere contributes to climate change, negatively impacting desert ecosystems by altering species distribution, reducing biodiversity, and affecting water availability. It is crucial to mitigate carbon emissions and promote sustainable practices to ensure the long-term stability and resilience of desert ecosystems.
- Q: How does carbon affect the pH of water bodies?
- Water bodies can be greatly influenced by the presence of carbon, which has the ability to alter their pH levels. When carbon dioxide from the atmosphere dissolves in water, it combines with water molecules to create carbonic acid. This natural process, known as carbonation, has a crucial role in regulating the pH of water bodies. The existence of carbonic acid in water has the potential to decrease its pH, resulting in increased acidity. This occurs because carbonic acid breaks down into hydrogen ions and bicarbonate ions. The higher the concentration of hydrogen ions, the lower the pH of the water, thus contributing to its acidity. Furthermore, carbonic acid can undergo further decomposition to form carbonate ions. These carbonate ions can react with hydrogen ions, ultimately reducing their concentration and raising the pH of the water. This process, called carbonation, acts as a buffer and aids in stabilizing the water's pH. Human activities, such as the combustion of fossil fuels and deforestation, release excessive amounts of carbon dioxide into the atmosphere. Consequently, this leads to an elevation in the concentration of carbonic acid in water bodies, resulting in a decrease in pH. This occurrence, known as ocean acidification, can have detrimental effects on marine life. The reduced pH caused by excess carbon can be harmful to aquatic organisms, particularly those with calcium carbonate shells, including corals, mollusks, and certain species of plankton. The acidic water dissolves their shells, rendering them more susceptible to predation and diminishing their ability to construct and maintain protective structures. In conclusion, the presence of carbon has a significant impact on the pH of water bodies due to the formation of carbonic acid. While carbonic acid contributes to water acidity, it also functions as a buffer and helps maintain pH stability. However, excessive carbon dioxide emissions resulting from human activities can lead to ocean acidification, which negatively affects marine life and the overall well-being of water ecosystems.
- Q: What are the long-term effects of increased carbon emissions on ecosystems?
- Increased carbon emissions have significant long-term effects on ecosystems. One of the most notable impacts is climate change, as carbon dioxide is a greenhouse gas that traps heat in the atmosphere. This leads to rising temperatures, altered weather patterns, and increased frequency and intensity of extreme weather events such as hurricanes, droughts, and wildfires. These changes in climate have numerous negative consequences for ecosystems. For instance, rising temperatures directly affect the physiology and behavior of plants and animals. Many species have specific temperature requirements for reproduction, feeding, and survival, and even slight changes can disrupt their life cycles and lead to population declines or extinctions. Furthermore, increased carbon emissions contribute to ocean acidification, a process where the excess carbon dioxide in the atmosphere dissolves in seawater, forming carbonic acid. This acidification has devastating effects on marine ecosystems, particularly coral reefs and shell-forming organisms like oysters and clams. It weakens their calcium carbonate structures and inhibits their growth and reproduction, ultimately leading to their decline. In addition, carbon emissions influence the distribution and composition of plant communities. As carbon dioxide is a vital component for photosynthesis, elevated levels can enhance plant growth and productivity. However, this can also lead to changes in plant composition and the competitive balance between species, favoring certain fast-growing species over others. This can disrupt the delicate relationships between plants and their pollinators, herbivores, and other organisms, affecting the entire food web. Moreover, increased carbon emissions contribute to the loss of biodiversity. Many species are highly specialized and adapted to specific environmental conditions. As habitats change due to climate change, certain species may struggle to adapt or find suitable alternatives, leading to declines or local extinctions. This loss of biodiversity can have cascading effects throughout ecosystems, disrupting ecological processes and reducing the resilience and stability of entire ecosystems. Overall, increased carbon emissions have far-reaching and detrimental long-term effects on ecosystems. They cause climate change, ocean acidification, alter plant communities, and drive biodiversity loss. It is crucial to reduce carbon emissions and mitigate climate change to protect and preserve the health and functioning of ecosystems for future generations.
- Q: How does carbon contribute to air pollution?
- Air pollution is primarily caused by carbon, which emits carbon dioxide (CO2) and carbon monoxide (CO) into the atmosphere. The burning of fossil fuels like coal, oil, and natural gas releases large quantities of carbon dioxide, a greenhouse gas responsible for global warming and climate change. This excess CO2 traps heat in the atmosphere, resulting in the greenhouse effect and a subsequent increase in global temperatures. Moreover, incomplete combustion of fossil fuels and biomass can release carbon monoxide, a toxic gas with harmful effects on human health. Carbon monoxide is especially dangerous because it binds to hemoglobin in the blood, reducing its ability to carry oxygen and potentially causing asphyxiation. Furthermore, carbon-containing compounds, such as volatile organic compounds (VOCs), also contribute to air pollution. These VOCs are released from various sources, including industrial processes, vehicle emissions, and the use of solvents in paints and cleaning products. When these compounds react with other pollutants in the atmosphere, they form ground-level ozone, a major component of smog. Inhaling ozone can lead to respiratory issues, eye irritation, and other health problems. In summary, carbon plays a significant role in air pollution by emitting carbon dioxide, carbon monoxide, and volatile organic compounds. These pollutants have profound impacts on climate change, human health, and the overall quality of the air we breathe. It is crucial to reduce carbon emissions and adopt sustainable practices to mitigate the adverse effects of carbon on air pollution.
- Q: How accurate is carbon dating?
- The scientific method known as carbon dating, or radiocarbon dating, is widely used to determine the age of organic materials that are up to 50,000 years old. It relies on measuring the ratio of radioactive carbon-14 (C-14) to stable carbon-12 (C-12) in a sample. Carbon dating has proven to be highly accurate, with a small margin of error. Its accuracy depends on factors such as the quality and preservation of the sample, the precision of measurement instruments, and understanding the carbon cycle in the past. However, carbon dating has limitations. It can only be used on organic materials that were once alive, so it is not applicable to dating inorganic materials like rocks or minerals. It is most effective for samples younger than 50,000 years old because the amount of C-14 decreases over time, making accurate measurement more challenging. To ensure accuracy, scientists often use multiple dating methods or cross-reference results with other independent techniques. This helps to verify the reliability of carbon dating and gain a more comprehensive understanding of the sample's age. Advancements in technology and calibration methods have improved the accuracy of carbon dating. For example, Accelerator Mass Spectrometry (AMS) allows for smaller sample sizes and greater measurement precision, reducing the margin of error. Calibration curves based on tree rings, or dendrochronology, also refine the accuracy of carbon dating. While carbon dating is highly reliable, it is important to recognize that no dating technique is perfect. All scientific dating methods have inherent limitations and uncertainties. However, with proper calibration and careful analysis, carbon dating remains one of the most accurate ways to determine the age of organic materials.
- Q: What are the effects of carbon dioxide on ocean acidity?
- Carbon dioxide (CO2) has a significant impact on ocean acidity, leading to a phenomenon known as ocean acidification. When CO2 is released into the atmosphere through human activities such as burning fossil fuels, it gets absorbed by the oceans. This absorption process triggers a series of chemical reactions that result in the formation of carbonic acid, which lowers the pH of the seawater. The increased concentration of carbonic acid in the oceans disrupts the delicate balance of carbonate ions, which are essential for the formation of calcium carbonate. Many marine organisms, including coral reefs, shellfish, and plankton, rely on calcium carbonate to build their shells and skeletons. As the ocean becomes more acidic, the concentration of carbonate ions decreases, making it increasingly difficult for these organisms to form and maintain their protective structures. Ocean acidification poses a significant threat to marine ecosystems and biodiversity. Coral reefs, for example, are particularly vulnerable to the effects of acidification. As the acidity increases, the coral's ability to build and maintain its calcium carbonate structure is compromised, leading to the bleaching and eventual death of the reef. This loss of coral reefs has severe consequences for the countless species that depend on these ecosystems for food, shelter, and reproduction. Furthermore, ocean acidification also affects other marine organisms, such as shellfish and plankton. Shellfish, including oysters, clams, and mussels, depend on calcium carbonate to form their shells. As the acidity rises, the availability of carbonate ions decreases, making it harder for these organisms to build their protective shells. This, in turn, can result in reduced populations of shellfish, impacting not only the organisms themselves but also the industries and communities that rely on them for economic and cultural reasons. Plankton, which are the foundation of the marine food web, are also susceptible to the effects of increased ocean acidity. Many plankton species have calcium carbonate structures that provide them with buoyancy and protection. As the acidity rises, these structures weaken, making it harder for plankton to survive and reproduce. This disruption in the plankton community can have far-reaching consequences for the entire marine food chain, impacting fish, marine mammals, and ultimately, humans who rely on seafood as a primary source of protein. In conclusion, the effects of carbon dioxide on ocean acidity are significant and alarming. Ocean acidification threatens the health and stability of marine ecosystems, impacting vital organisms like coral reefs, shellfish, and plankton. Understanding and addressing this issue is crucial for the long-term health of our oceans and the countless species that depend on them.
- Q: How does carbon affect air quality?
- Carbon can have both positive and negative effects on air quality. On one hand, carbon dioxide (CO2) is a natural component of the Earth's atmosphere and is necessary for the survival of plants and photosynthesis. However, excessive amounts of CO2 can contribute to the greenhouse effect, leading to global warming and climate change. Additionally, carbon monoxide (CO), a byproduct of incomplete combustion, is a harmful pollutant that can negatively impact air quality. It is primarily emitted from vehicles, industrial processes, and residential heating systems. High levels of carbon monoxide can impair the delivery of oxygen to the body, leading to various health issues, including headaches, dizziness, and in extreme cases, even death. Furthermore, carbon-containing compounds such as volatile organic compounds (VOCs) can contribute to the formation of ground-level ozone, a harmful pollutant. Ground-level ozone can cause respiratory problems, aggravate existing respiratory conditions, and reduce lung function. VOCs are emitted from various sources, including vehicle emissions, industrial processes, and certain household products. In conclusion, while carbon dioxide is essential for life on Earth, excessive amounts can contribute to climate change. On the other hand, carbon monoxide and volatile organic compounds emitted from human activities can negatively impact air quality and human health. Therefore, it is crucial to reduce carbon emissions and adopt cleaner technologies to mitigate the adverse effects of carbon on air quality.
- Q: What are the consequences of increased carbon emissions on global food security?
- Increased carbon emissions have significant consequences on global food security. Firstly, rising carbon dioxide levels can lead to changes in temperature and precipitation patterns, affecting crop productivity and water availability. This can result in reduced yields, crop failures, and increased vulnerability to pests and diseases, ultimately impacting food production and availability. Furthermore, carbon emissions contribute to climate change, which exacerbates extreme weather events like droughts, floods, and heatwaves. These events can destroy crops, disrupt supply chains, and increase food prices, making it difficult for vulnerable populations to access nutritious food. Additionally, climate change may lead to the loss of arable land due to desertification, sea-level rise, or other environmental changes, further diminishing food production capacity. Moreover, carbon emissions contribute to ocean acidification, which harms marine ecosystems and disrupts the food chain. This can negatively impact fish stocks and other seafood sources, affecting the livelihoods of coastal communities who rely on fishing as a primary source of food and income. Overall, increased carbon emissions have severe consequences for global food security, threatening the stability and accessibility of food supplies both on land and in the oceans. Addressing carbon emissions and adopting sustainable practices are essential in safeguarding our food systems and ensuring the wellbeing of future generations.
- Q: How does carbon affect ocean acidification?
- Various human activities, such as burning fossil fuels and deforestation, release carbon dioxide (CO2) into the atmosphere. This CO2 is a greenhouse gas that, when absorbed by the oceans, leads to a process called ocean acidification. When CO2 dissolves in seawater, it reacts with water molecules and forms carbonic acid. This reaction increases the concentration of hydrogen ions (H+), resulting in a decrease in pH levels and making the seawater more acidic. This decrease in pH is a key characteristic of ocean acidification. As the ocean becomes more acidic, it disrupts the delicate chemical balance that many marine organisms rely on for survival and growth. Organisms like corals, shellfish, and phytoplankton use calcium carbonate to build their shells or skeletons, but increased acidity hampers their ability to do so. Ocean acidification also impacts the growth and development of marine plants and animals. For instance, changes in pH levels can affect the ability of larvae from certain marine species to form strong shells or skeletons. Additionally, acidified waters can disrupt the metabolism and reproductive processes of many marine organisms. The consequences of ocean acidification extend beyond individual organisms. Entire ecosystems, such as coral reefs, face threats due to increasing acidity. Coral reefs provide habitat for numerous species and are vital to marine biodiversity. However, the more acidic conditions make it challenging for corals to build and maintain their calcium carbonate structures, resulting in coral bleaching and degradation of reef systems. Moreover, ocean acidification can have cascading effects on other marine organisms and food webs. For example, changes in the growth and survival rates of phytoplankton, a primary food source for many marine species, can disrupt the entire food chain, impacting fish populations and ultimately affecting human communities that rely on seafood for sustenance and livelihoods. In conclusion, the rise in carbon dioxide emissions contributes to ocean acidification, which alters the chemistry of the oceans and poses significant threats to marine life and ecosystems. Understanding and addressing the causes and impacts of ocean acidification are essential for the long-term health and sustainability of our oceans.
- Q: What are the applications of graphite in industry?
- Graphite possesses distinct properties that make it suitable for a range of applications across industries. Here are several key uses of graphite in different industrial sectors: 1. Lubricants: Given its low friction coefficient, graphite is extensively employed as a solid lubricant in industries that encounter high temperatures and extreme pressures, like automotive, aerospace, and heavy machinery. 2. Refractories: Graphite's exceptional heat and chemical resistance make it an ideal material for manufacturing refractory products. It helps line furnaces, crucibles, and other high-temperature equipment in metal production, glass manufacturing, and chemical processing. 3. Electrical industry: Graphite's excellent electrical conductivity makes it widely utilized in this sector. It is employed to produce electrodes, brushes, and contacts for electrical motors, generators, and batteries. Furthermore, graphite serves as a component in electrical discharge machining (EDM) and conductive paints and coatings. 4. Foundry industry: Graphite acts as a mold and core material in the foundry industry, owing to its high thermal conductivity and ability to withstand high temperatures. It finds application in various metal casting processes, including sand casting, investment casting, and continuous casting. 5. Chemical industry: The chemical industry benefits from graphite's corrosion resistance and capacity to endure high temperatures. It is utilized in the manufacture of chemical equipment such as heat exchangers, reactors, and pipes, where it can withstand aggressive chemical environments. 6. Nuclear industry: In the nuclear industry, graphite serves as a moderator in nuclear reactors. Its ability to slow down neutrons allows for controlled nuclear fission reactions. Additionally, graphite is employed as a structural material in certain types of nuclear reactors. 7. Composite materials: Graphite is frequently used as a reinforcement material in the production of composite materials. By combining graphite fibers or sheets with resins or metals, lightweight and high-strength composites are created for applications in aerospace, automotive, and sporting goods industries. In conclusion, graphite's unique properties, encompassing high thermal and electrical conductivity, lubricity, and chemical inertness, contribute to its versatility as a material with diverse applications across industries.
Send your message to us
Low Sulfur Calcined Petroleum Coke of CNBM in China
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 1 m.t.
- Supply Capability:
- 10000000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords