HPMC/MHPC Cellulose Ether Cement-based Mortar
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5000 kg
- Supply Capability:
- 5000000 kg/month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Building Construction Hydroxypropyl Methyl Cellulose (HPMC)
Brief introduction:
Hydroxypropyl Methyl Cellulose (HPMC) helps building materials apply more easily and perform better. They provide water retention and cohesiveness to mixtures. With special modification, it can be used to control thickening, water demand, workability, sag resistance, strength and other important properties of the final product.
It is widely used as thickener, adhesive, water preserving agent, film-foaming agent in building materials, industrial coatings, synthetic resin, ceramic industry, medicine, food, textile, agricultural, cosmetic and other industries.
Physical and chemical index:
Item | Specification |
CAS NO. | 9004-65-3 |
Appearance | white or light yellow powder |
Moisture Content | ≤5.0% |
PH | 4.0-8.0 |
Particle Size | min. 98% pass through 100 mesh |
Viscosity | 100cps-200000cps, 2% solution |
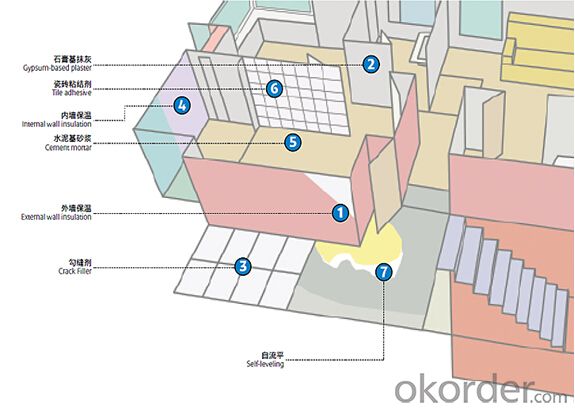
Application in Building:
External wall insulation system (EIFS)
>Bond strength: HPMC can provide the greatest degree of high adhesive bond strength of mortar.
>Performance: The mortar added HPMC has the right consistency, non-sagging. When using, the mortar is easy to work continuously, uninterrupted.
>Water retention: HPMC can wet the wall insulation easily, easy to paste, and also make other additional materials reach the best affects.
>Absorbent: HPMC can minimize the air-entraining volume, lower water absorption of mortar.
>Recommended brand: 75CMAX75000(S), 75CMAX100000(S), 75CMAX200000(S)
Interior and exterior wall interface agent
>Easy to mix, without of agglomeration: HPMC can significantly reduce the friction in the dry powder during the process of mixing with water, which makes it easy to mix and save the blending time.
>Water retention: HPMC can significantly reduce the moisture absorption by the wall. Good water retention can ensure the cement compound with a longer time, also can ensure that workers are able to carry out many times of scraping for the putty on the wall.
>Good working performance stability: even in high temperature environment, HPMC can still maintain good water retention. it is suitable for construction in the summer or hot areas.
>Increased water demand: HPMC can significantly improve the water demand of the putty materials. On the one hand, it improves the operational time after putty put on the wall, on the other hand, it can increase the coating are of the putty, which can make the formula more economical.
>Recommended brand: 75CMAX60000(S), 75CMAX75000(S)
Tile adhesive
>Water retention: HPMC can reduce the moisture absorbed by the substrate and the tile, retain the moisture in the adhesives as much as possible, making mortar still have adhesion after coating for a long time. Significantly extend open time and makes bigger coating area for the worker each time, and improve the efficiency.
>Improve bond strength, improve anti-slip performance: HPMC ensure non sagging of the tiles during working, especially for heavy tile, marble and other stone materials.
>Work performance: The lubricity of HPMC can increase the workability of the mortar significantly, which makes the mortar easy to coating and improve efficiency.
>Improve mortar wetting property: HPMC give mortar consistency, enhance the wetting ability of mortar and substrate, increase the binding strength of wet mortar, especially for the recipe with high water cement ration;
>Recommended brand: 75CMAX40000(S), 75CMAX75000(S), 75CMAX100000(S)
Crack Filler
>Workability: provide the right viscosity, plasticity, and easy to work;
>Water retention: can make the slurry fully hydrated, extending the working time and avoid cracking.
>Anti-hanging: HPMC can make a strong adhesion on the surface for the slurry and not sag;
>Recommended brand: 75CMAX40000(S), 75CMAX75000(S), 75CMAX100000(S)
Self-leveling mortar
>Prevent bleeding: HPMC can play a very good role to prevent the slurry sedimentation, bleeding.
>Maintain liquidity, and improve retention: low viscosity HPMC will not affect the slurry flow effect and easy to work. While possesses certain water retention, makes the good surface effect after self-levelling and avoid cracks.
>Recommended brand: 75CMAX400~600
Gypsum-based plaster
>Water retention: HPMC can retain moisture in the mortar, thus make gypsum completely solid. The higher the viscosity is, the stronger the water-retention capacity, vice versa..
>Sag resistance: allow the worker make the thick coating without causing ripple building.
>Mortar yield: For fixed weight of dry mortar, the exist of HPMC can provide more wet mortar.
>Recommended brand: 75CMAX75000(S), 75CMAX100000(S)

FAQ
Q1.Could we have the sample to test the quality?
Kindly send us your address, we are honored to offer you samples.
Q2. How does your company do regarding quality control?
CNBM a Chinese state-owned enterprise ranked 270th among the global fortune 500 in 2015,
have accreditation in line with standard:ISO 9001:2000,SGS,CIQ certificate.
Q3:What's your Delivery Time?
In generally, the delivery time is 25 days-30 days.We will make the delivery as soon as possible with the guaranted quality.
Q4:What is the convenient way to pay?
L/C , T/T ,Paypal, Western Union and Escrow are accepted,and if you have a better idea , please feel free to share with us .
Q5:Which mode of transport would be better?
In general,we advice to make delivery by sea which is cheap and safe.Also we respect your views of other transportation as well.
- Q: A biological catalyst or a chemical reaction facilitator is know as a/an?
- A biological catalyst is an enzyme. Here are more details for you. Enzymes – biological catalysts Normally chemical reactions do not proceed spontaneously, but require the help of a catalyst. A catalyst accelerates a chemical reaction without itself being changed. For example, the reaction of hydrogen with oxygen to produce water requires the addition of the metal platinum. These days we encounter the concept of a catalyst most often in connection with technology for cleaning up the exhaust fumes from our automobiles, where platinum and rhodium catalyze the breakdown of polluting nitrogen oxides. Chemical reactions within living cells must also be catalyzed. Biological catalysts are called enzymes. There is, for instance, an enzyme in our saliva which converts starch to a simple sugar, which is used by the cell to produce energy, and another enzyme which degrades the excess lactic acid produced when we overexert ourselves. All green plants contain enzymes which convert carbon dioxide in the air to nutritious carbohydrates such as sugar and starch. Without enzymes life would not be possible! Enzymes are highly selective. Among the thousands of different compounds in a cell, an enzyme can recognize the right molecule (substrate) and transform it into a new product. This property arises from the special three-dimensional structure of each enzyme. One can compare an enzyme and its substrate with a lock and its key. Enzymes are very effective catalysts. A chemical reaction might require several months to reach completion without a catalyst, but only a few seconds with the help of an enzyme. Since the enzyme remains unchanged, one enzyme molecule can catalyze the transformation of millions of substrate molecules. Up until the beginning of the 1980's, all enzymes were thought to be proteins. We now know that proteins do not have a monopoly on biocatalysis. RNA molecules can also function as enzymes.
- Q: What are the examples of chemical catalysts used in life?
- Clothing. "New synthetic fiber made of clothing, soft and comfortable and cheap and durable. Cloth from natural fibers to man-made fibers, and then to the development of synthetic fibers, dyes from the original natural dyes to the current synthetic dyes, reactive dyes , All reflect the contribution of chemistry to the development of clothing, chemical clothing from the initial cover utility, into today's beautiful, convenient, with a special function of the utility, it greatly enriched the style of clothing, material, use
- Q: what is the role of a catalyst in a chemical reaction?
- It increases the rate of reaction by lowering the requirement of energy needed to carry out the chemical reaction. Hope that helped.
- Q: The role and significance of chemical catalysts
- To speed up or slow down the chemical reaction is to make the chemical reaction more direct, simple and straightforward to adopt
- Q: And hydrogen peroxide
- Yes, as long as it is copper ions and iron ions on the line, such as FeCI can
- Q: Can chemical reaction limits be changed by catalyst or other methods?
- No, the catalyst can only speed up the chemical reaction, but can not change the chemical limit.
- Q: How to poison the catalyst. What can be done?
- In the reactants or catalyst mixed with a small amount of material, so that the catalyst catalytic capacity of a sharp decline or even loss, this phenomenon is called catalyst poisoning. For example, in the synthesis of ammonia feed gas containing CO, CO2 and H2S, PH3, water vapor and other impurities, can make iron catalyst poisoning; contact with the system of sulfuric acid, if arsenic and selenium oxide (As2O3, SeO2), can make vanadium catalyst Loss of activity. Therefore, it is necessary to purify the feed gas, prevent the poisoning of the catalyst, and also reduce the corrosion of the equipment. The phenomenon of catalyst poisoning is sometimes temporary, the removal of toxicants, the effectiveness of the catalyst can still be restored; sometimes it is permanent, without chemical treatment can not restore catalytic performance.
- Q: In the chemical reaction, the rate of decomposition reaction is related to the quality of the catalyst?
- The catalyst can affect the reaction rate, the faster the amount of reaction or slower. Of course there are limits,
- Q: chemistry subject
- A catalyst is a substance which alters the rate of a chemical reaction but is chemically unchanged at the end of the reaction there are well some of em are 2,2'-Bis(2-indenyl) biphenyl Adams' catalyst Band 3 Cerium(IV) oxide Copper(II) acetate Copper(II) hydroxide Cyclooctadiene rhodium chloride dimer 4-Dimethylaminopyridine Enzyme engineering Faujasite Frustrated Lewis pair Grubbs' catalyst Hopcalite Incipient Wetness Impregnation Lanthanide triflates Lindlar catalyst Mesoporous silicates Methylaluminoxane NOBIN Nickel(III) oxide Noxer block Palladium on carbon Phase transfer catalyst Platinum Polyoxometalate P cont. Post-metallocene catalyst 2-Pyridone Pyrotol catalyst Raney nickel Ribozyme Tetrakis(triphenylphosphine)palladium(... Tetrakis(triphenylphosphine)platinum(0... Triazabicyclodecene Tris(dibenzylideneacetone)dipalladium(... Trost ligand Vanadium(V) oxide Wilkinson's catalyst Ziegler-Natta catalyst
- Q: Can you describe at least 4 ways a catalyst can lower the activation energy of a reaction?
- To see how a catalyst accelerates the reaction, we need to look at the potential energy diagram shown below which compares the non-catalytic and the catalytic reaction. For the non-catalytic reaction, the figure is simply the familiar way to visualize the Arrhenius equation: the reaction proceeds when A and B collide with succificient energy to overcome the activation barrier. The change in Gibbs free energy between reactants, A + B, and the product P is delta G. The catalytic reaction starts by bonding of the reactants A and B to the catalyst, in a spontaneous reaction. Hence, the formation of this complex is exothermic and the free energy is lowered. There then follows the reaction between A and B while they are bound to the catalyst. This step is associated with an activation energy; however, it is significantly lower than that for the uncatalyzed reaction. Finally, the product P seperates from the catalyst in an endothermic step. The energy diagram illustrates 4 ways the catalyst works : The catalyst offers an alternative path for the reaction that is energetically more favorable The activation energy of the catalytic reaction is significantly smaller than that of the uncatalyzed reaction; hence the rate of the catalytic reaction is much larger The overall change in free energy for the catalytic reaction equals that of the uncatalyzed reaction. Hence, the catalyst does not affect the equilibrium constant for the overall reaction. A catalyst cannot change the thermodynamics of a reaction but it can change the kinetics. The catalyst accelerates both the forward and the reverse reaction to the same extent. In other words, if a catalyst accelerates the formation of product P from A and B, it will do the same for the decomposition of P into A and B.
Send your message to us
HPMC/MHPC Cellulose Ether Cement-based Mortar
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5000 kg
- Supply Capability:
- 5000000 kg/month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords






















