Cyanuric Acid Lead Granular Tablets Powder Standrd Quality
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 17 m.t.
- Supply Capability:
- 1800 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Cyanuric Acid
The Structure of Cyanuric Acid Descriptions:
Trade Name: Isocyanuric Acid
Other name: Cyanuric Acid; 1,3,5-Triazine-2,4,6-triol
Uses: Bleaches and sanitisers.
Formula: C3H3N3O3
Molecular Weight: 129.07
CAS NO.: 108-80-7
Main Features of Cyanuric Acid:
White powder, granular or colored tablet form, non-toxic and odorless
Full experience of large numbers containers loading in Chinese sea port
Fast shipment by reputed shipping line
Packing with pallet as buyer's special request
Best service after shipment with e mail
Cargoes together with container sales seervice available
Full experience for Canada & Japan export
Cargoes photo before and after loading into container
Raw materials from chinese origin
Cyanuric Acid Specification:
| ITEM | SPECIFICATION | RESULT | |||
| Content | ≥98.5% | 98.64% | |||
| Moisture | ≤0.5% | 0.11% | |||
| PH value | 4.0-4.5 | 4.26 | |||
| Fe2+ | ≤15ppm | 7.5ppm | |||
| NH4+ | ≤200ppm | 97ppm | |||
| Ash | ≤0.1% | 0.05% | |||
| Insoluble matter in DMF | ≤0.3% | 0.25% | |||
| Appearance | White crystalline power | White crystalline power | |||
| Mesh number | 95% pass 80 mesh | 95% pass 80 mesh | |||
| White degree | ≥89 | 90.5 | |||
| Conclusion: | The product complies with the standard above. | ||||
Cyanuric Acid Packing:
in 25kg, 1000kg bag for powder
in 25kg plastic bag or 50kg PE drums for granular

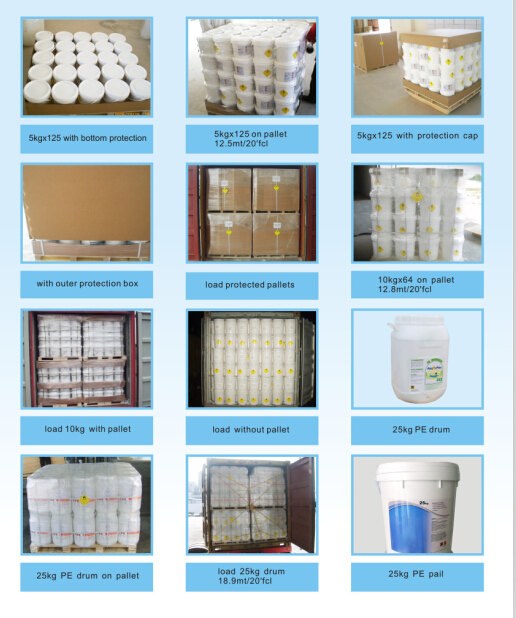

Service we can provide:
1. Mixed container, we can mix different items in one container.
2. Quality control, before shipment, free sample for test. after shipment, keep sample for 3 years
3. Prompt shipment with professional documents
4. Packing as your request, with photo before shipment
Professional Loading:
1.We will provide you with professional loading
2.We have one team supervise uploading the materials. We will check the container, the packages
3.Every step, taking pictures and make records.
4.we will make a complete Loading Report for our customer of each shipment
- Q: What are the examples of chemical catalysts used in life?
- The use of new synthetic materials makes life more comfortable. Only wood, sand and grapes are natural building materials, but they need to be combined and protected with synthetic chemicals. Cement is a chemical product, As the adhesive used in the laminate and the metal used in the nail are chemical products, the glass is made by the chemist, and the improved product, such as heat-resistant glass (trade name Pyrex glass), becomes more tough. Paint is chemist design and creation, and many modern solid materials are also the same. Plastic is synthetic, they are used in kitchen and bathroom utensils, also used in the name of the product called Formica bakelite and its related materials, beverage bottles, Cutlery and utensils. Porcelain is made by chemists and used in kitchen and bathroom sinks and other fixtures. Metal is made of chemical changes made from ores. Aluminum was once a laboratory treasure, but used An electrochemical method, which now can be easily made from alumina, at least a portion of the carpet and decorative fabric used for the use of synthetic fibers and synthetic dyes to color. Freezer and air conditioner with special chemicals as coolant ; Gas and gas stoves can be used syngas or natural gas, the combustion process is still the chemical change.Our room with gas or oil industry to produce fuel to heat, this fuel is from the natural crude oil refining and chemical We have made use of synthetic chemical products and materials made in the chemical processing industry, such as plaster or wall panels, outer panels and roof panels, as well as tiles and carpets, to heat our buildings. The stove itself and the distribution of heat The pipes are made of chemical products - metal, insulating materials and ceramics. The current enters the home through the copper wire of the outsourced insulator, both of which are products of the chemical processing industry
- Q: How does the catalyst affect chemical balance? Why the catalyst has no effect on the chemical equilibrium, for v-t diagram
- Negative catalyst increases the activation energy, so that the reaction time becomes longer
- Q: Is the catalyst in the field of inorganic chemistry?
- In particular, the chemical and homogeneous catalysis of inorganic chemistry has deep origins. Inorganic chemistry, oxides (such as metal oxides), family elements (such as the chemical behavior of transition metal elements) can be provided for catalytic science Support and guidance.
- Q: Please help me
- A catalyst is a substance which is used to increase or decrease the rate of a reaction, without itself undergoing any chemical change. There are two types of catalysts, positive and negative. Positive catalysts are used to increase the rate of a reaction while negative catalysts are used to decreasing the rate of a reaction. Enzymes are proteins which act as catalysts in biochemical reactions. They operate between a certain pH level and temperature. If there is a change in pH level or temperature, their efficiency decreases.
- Q: What is the quality of the catalyst in the chemical reaction, for example, 34.3 g before the hydrogen peroxide reaction, 32.7 g after the reaction, and how much is the catalyst mass?
- You can not calculate this question, the quality of the catalyst before and after the same reaction, how much reaction before the reaction on how much
- Q: Is the chemical reaction rate constant related to the amount of catalyst used?
- The catalyst has a certain amount of suitable range, the general factory production of some substances (such as ammonia), the amount of catalyst used is limited, to achieve a value after no greater role. So the reaction rate constant is independent of the amount of catalyst used
- Q: CO and NO react under the action of a catalyst to generate chemical formulas for CO2 and N2.
- Landlord's question should not know how to match it, with the loss of electronic with a constant match
- Q: Chemical master invited (about catalyst)
- From the thermodynamics can be reaction, and the three formulas can be added to eliminate the intermediate product, indicating that the reaction may occur. The definition of the catalyst is not complete. I am a junior undergraduate student of Jilin University School of Chemistry, according to the definition of the catalyst in the university textbook, the catalyst itself reacts with the reactants to produce unstable intermediates. After the reaction is finished, the intermediate product is explained and the catalyst is reduced. Apparently did not participate in the reaction. So the catalyst to change the course of the reaction, the original reactants to go through a relatively high energy to produce products, there will be a catalyst after a few relatively low energy barrier, so much easier, the reaction rate is greatly accelerated The It can be seen, the catalyst is not no response, but only after the completion of the reaction to restore it. It can also be seen that the amount of catalyst does not matter, and some reactions require the amount of catalyst to be approximately equal to the amount of reactants. Waiting for you to high school and university to further study on this issue will have a more clear understanding of the.
- Q: Palladium is the main catalyst in chemistry?
- Palladium in the chemical mainly to do the catalyst; palladium and ruthenium, iridium, silver, gold, copper and other alloy, can improve the palladium resistivity, hardness and strength, used in the manufacture of precision resistors, jewelry and so on. While the most common and most commercially available palladium jewelery is palladium.
- Q: Why does the CuO catalyze the reaction rate faster and faster when catalyzing the decomposition of hydrogen peroxide or tell me how to make the catalyst catalyst faster
- CuO exothermates when catalyzing the decomposition of hydrogen peroxide, so the reaction becomes faster.
Send your message to us
Cyanuric Acid Lead Granular Tablets Powder Standrd Quality
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 17 m.t.
- Supply Capability:
- 1800 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords

























