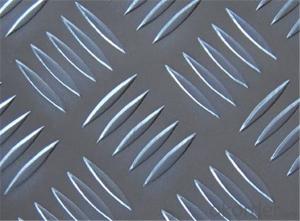
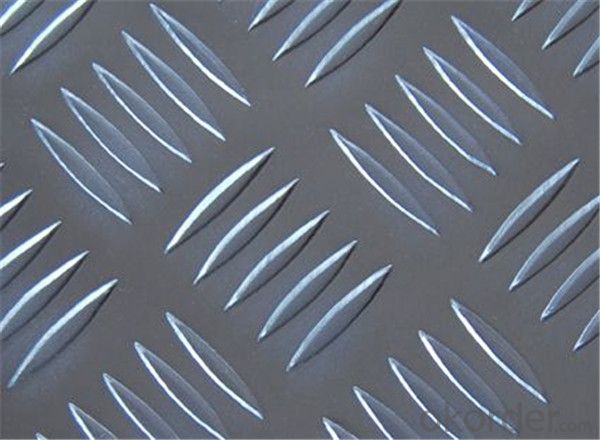
Amcor Aluminum Foil - Aluminium Stucco Embossed Sheet 5052 1.2mm Thickness
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 5000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Packaging & Delivery
| Packaging Details: | export standard packing |
| Delivery Detail: | within 15-25 days after receipt of your deposi |
Specifications
stucco embossed aluminum sheet
alloy: 1100, 1060, 3003, 8011
thickness 0.02-1.5mm
width 200-1200mm
Stucco Embossed Aluminum Coil
1.Introduction of embossed sheet
| embossed aluminium sheets | |||||
| PRODUCT | ALLOY | TEMPER | THICKNESS | WIDTH | APPLICATIM |
| stucco pattern | 1050 1060 1100 1100 3003 3004 3105 5005 5052 | O H14 H16 H18 H22 H24 H32 | 0.01-3.0mm | 800-1226mm | automobile, airplane, shop, light rail, vehicle steps, stair treads, elevators, etc |
| semi-circle ball | 0.1-1.0mm | 800-1220mm | |||
5 bars, tread plate diamond pattern | 0.9-8.0mm | 800-1600mm | |||
| diamond pattern | 0.1-2.0mm | 800-1220mm | |||
2.The base info. of embossed aluminum sheet:
1) Alloy of aluminum sheet: mainly 1100, 1050, 1060,3004,3005
2) MOQ: 3 tons
3) Delivery time: 25 days
4) Pattern: embossed, diamond, worm, pointer, 5-bar, 3-bar
3.Advantage of aluminum embossed sheet:
1)high temperature resistant
2)weathering resistant
3)scrubbing resistant
4)sound insulation
5)acid or alkali proof
6)fireproof
7)light weight material is easy to construct and install
4.Appearance finishing quality aluminum stucco embossed sheet:
| 1) Chemical ingredients: | According with the rules of GB /T3190 |
| 2) Physical property: | The physical property testing of basis material under room |
| Temperature accords with the rules of GB /T8544-1997 | |
| 3) Appearance quality: | Clear patern and tidy without burr |
| 4) Surface: | No crack, selvedge, canker, hole |
Photos




- Q: I am the tree hugger, and I like to take care of the environmentI want more ways to take care of the environment.
- that is a myth, the only difference might be how much heat is reflectedGo to Discovery or Metacafe and type How it is made, aluminum foil and you will see why it is a myth.
- Q: What is the aluminum foil?
- A shielding film, with more in the electronics industry, to shield the disturbance effect, is the commonly used single blue aluminum foil,
- Q: how many number of valence of electrons are in hydrogen, sodium, strontium, boron, phosphorus, chlorine, neon, lithium, aluminum, sulfur, krypton, iodine, beryllium, nitrogen, helium, potassium, barium, xenon, and oxygen????? thanks so much you guys:D
- look on the periodic table and see what columns they are in1st column has one valence electron, etc.
- Q: how to cook lasagna?
- First I wash my hands, then I heat up some water to soften up the lasagna but not to softThen in a glass baking dish I will lightly coat the bottom with Olive Oil then I will place a layer of the pasta, then the Meat sauce (Store bought is fine) then some Mozzarella Cheese, if you also like you can add some canned mushrooms and repeat this 3 to 4 timesThen you can bake it on a Medtemp maybe 320 degrees till your pasta is firmly cookedI know this isn't traditional but its quick and easy, especially if you just got home tired from work.
- Q: I often times look at my current situations as a chance to play games with the current fadsMy current game seems to be that I've been quot;greenquot; my whole life, but just now its become a popular thing.I was needing an outside opinionJust how green on a scale of 1-10 am I?I recycle steel, aluminum, platic bottles, and news papers.I use old paper egg cartons and make my own paper and christmas cardsI reuse old christmas boxes again and againI only buy phosphate free soaps and detergentsNo Incandessant bulbsOnly day light and CFLS.I freecycleMake my own household cleaners out of Vinegar Baking Soda and BoraxIf its Yellow Let it mellow.Energy Star TV and Computer ( I'm a renter so now choice with other appliances)My car gets 33mpgI make it habit to not buy Greenwashed products without combing the ingredients.Thats just a glimpseIf you can rate me I'd appreciate itSuggestions may be the best answer10 points for the best suggestions
- Check the last equationShould be .169.86 cm2 So, now divide the 0.38 cm3 by the 16.86 cm2 and you get the answer in cm There is a little of something called dimensional analysis in thereAs in cm3/cm2cmA good help in checking if you divided the right things in the right order.
- Q: Smoking using gas grill: Remove Burner Shield or not?
- They build a platform in the water and lower the car by helicopter on to it.
- Q: I can't figure out the electron configurations of Aluminum, Argon, or Boron.Any help is appreciated.
- Boron : Atomic Number 5 Electron Configurations 1s2 ; 2s2 ; 2p1 or 1s2 2s2 2p1 Aluminum : Atomic Number 13 Electron Configurations 1s2 ; 2s2 ; 2p6 ; 3s2 ; 3p1 or 1s2 2s2 2p6 3s2 3p1 or [Ne] 3s2 3p1 Argon : Atomic Number 18 Electron Configurations 1s2 ; 2s2 ; 2p6 ; 3s2 ; 3p6 or 1s2 2s2 2p6 3s2 3p6 or [Ne] 3s2 3p6 [Ne] means - Configuration of the first ten electrons are the same as in Neon Atom ( 1s2 2s2 2p6 )
- Q: For my engineering class, I need to make an aluminum foil boat out of a 12quot; by 12quot; piece of aluminum, 7 straws, and scotch tapeIt needs to hold 100 pennies, and I'm not sure how to accomplish this, any help would be great :DThanks
- Determine the weight of 100 penniesSince the density of water is about 1 gram per cubic centimeter (cc), the weight in grams is the volume of water your boat must displaceEureka! - see Archimedes
- Q: 5 drops of aluminum nitrate is placed in a centrifuge tube6 drops of aqueous ammonia solution is then addedA precipitate forms.Explain what happenedWhat is the chemical identity of the precipitate? (hint: What IONIC species arise when NH3 is added to water? The precipitate forms because Al(3+) has combined with an anion.)Write TWO net ionic equations.
- Reaction 1 NH3 + H2O - NH4+ + OH- The ammonia in water turns the water solution basic Reaction 2 Al+3 + 3OH- - Al(OH)3 (s) Aluminum hydroxide is an insoluble precipitate.
- Q: be produce?Please show me the steps and explain for me so I can understand it and try to teach it to myself :)
- 200 grams of raw material couldn't produce more than 200 grams of productnewtons laws.
Send your message to us
Amcor Aluminum Foil - Aluminium Stucco Embossed Sheet 5052 1.2mm Thickness
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 5000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords