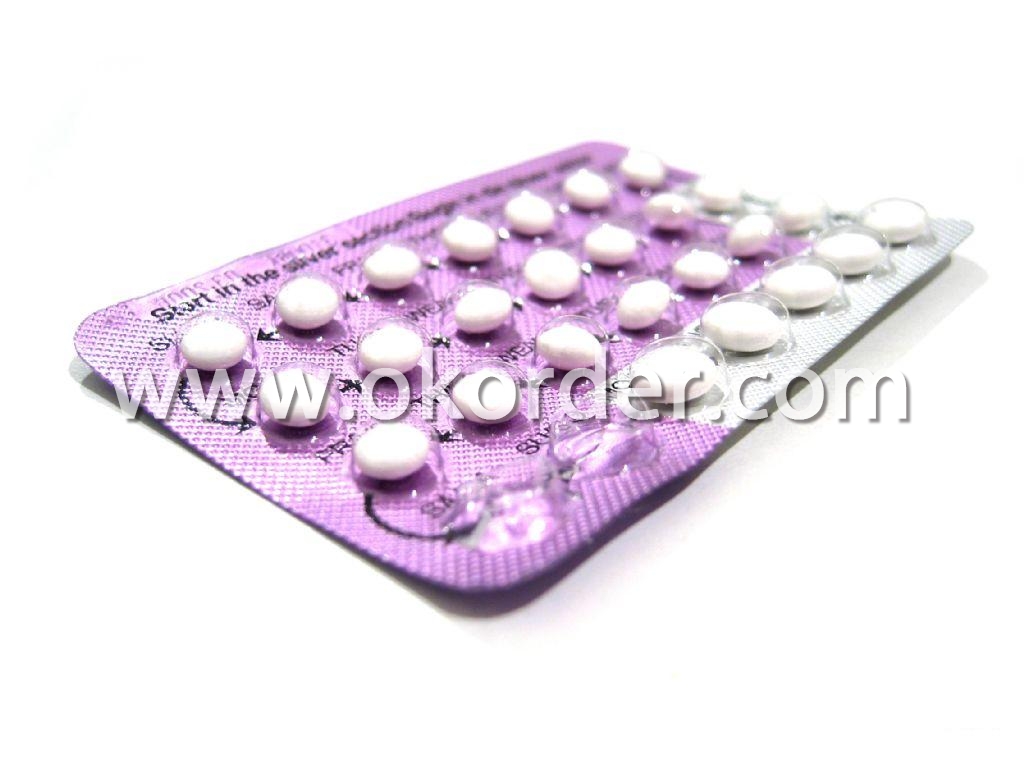
Aluminum Foil for Blister Packs
- Loading Port:
- China Main Port
- Payment Terms:
- TT or LC
- Min Order Qty:
- 1 m.t.
- Supply Capability:
- 7000MT/MONTH m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Quick details of Aluminium Foil For Blister
Alloy: 8011/8006/8079
Temper: H18/O
Specifications of Aluminium Foil For Blister
Thickness & Tolerance: 0.02mm-0.09mm (±6%)
Width & Tolerance: 200 - 1650 mm (+/-1 mm)
Mechancial Properties: Tensile Strength(U.T.S)≥160Mpa, Elongation≥1%
Standard: GB/T3198 / ASTM-B209 / EN546
Usage/Application of Aluminium Foil For Blister
For PTP foil, cord-forming foil, alu-alu foil
Packaging & Delivery of Aluminium Foil For Blister
Packing: seaworthy wooden box with pallet
Delivery: to be loaded by 1 x 20 feet container

- Q: Is it possible to utilize aluminum as a material for manufacturing solar cells?
- <p>Aluminum is not typically used to make solar cells due to its properties. Solar cells are usually made from materials like silicon, which is a semiconductor and can efficiently convert sunlight into electricity. Aluminum, being a good conductor, is used in the manufacturing process of solar cells for its electrical conductivity, but not as the active material that generates electricity. It is used in the form of aluminum paste in the back surface field of solar cells to create a conductive layer and in the frame to provide structural support. However, for the photovoltaic conversion process itself, other materials are more suitable.</p>
- Q: This question asks for an enumeration of different types of aluminum alloys.
- <p>Aluminum alloys are categorized based on their primary alloying elements and their applications. Some common types include: 1) 1000 series (99% pure aluminum), 2) 2000 series (alloyed with copper), 3) 3000 series (alloyed with manganese), 4) 4000 series (alloyed with silicon), 5) 5000 series (alloyed with magnesium), 6) 6000 series (alloyed with magnesium and silicon), and 7) 7000 series (alloyed with zinc). These alloys have various properties, making them suitable for different industries such as aerospace, automotive, and construction.</p>
- Q: Explain the interaction between aluminum and oxygen.
- <p>Aluminum and oxygen have a reactive relationship. When aluminum is exposed to oxygen, it forms a thin, protective layer of aluminum oxide on its surface. This oxide layer prevents further oxidation of the aluminum beneath it, making aluminum a corrosion-resistant metal. The reaction is exothermic and can be represented by the chemical equation: 4Al + 3O2 鈫?2Al2O3. This aluminum oxide layer is hard and stable, which is why aluminum is widely used in various applications where resistance to corrosion is important.</p>
- Q: This question asks about the safe usage of aluminum in everyday life.
- <p>Aluminum is widely used in daily life due to its lightweight and corrosion-resistant properties. To use aluminum safely, ensure that aluminum cookware is not used for acidic foods as it may leach aluminum into the food. Avoid storing acidic beverages like soda in aluminum cans for extended periods. Use aluminum foil in moderation, especially when cooking at high temperatures, as it can release aluminum into food. Always wash aluminum utensils thoroughly before and after use. For aluminum cans, recycle them to reduce environmental impact and avoid ingestion of aluminum particles from damaged cans.</p>
- Q: Is it possible to utilize aluminum in the manufacturing of medical devices like stents or implants?
- <p>Aluminum is not typically used in medical devices such as stents or implants due to its potential toxicity and lack of biocompatibility. Medical-grade materials like stainless steel, titanium, and various biocompatible polymers are preferred for their strength, durability, and ability to be safely integrated with human tissues. Aluminum can cause adverse reactions in the body, and its use in medical devices is generally avoided to prevent such complications.</p>
- Q: Can recycled aluminum be utilized in the production of solar panels and other renewable energy technologies?
- <p>Yes, recycled aluminum can be used to make solar panels and other renewable energy sources. Aluminum is a key component in solar panel frames and mounting structures due to its lightweight, high strength, and corrosion resistance. Recycling aluminum saves energy and resources compared to mining and refining new aluminum. Additionally, recycled aluminum can be used in wind turbines, hydroelectric generators, and other renewable energy technologies where its properties are beneficial. The use of recycled materials in renewable energy production contributes to a more sustainable and circular economy.</p>
- Q: This question asks about the consequences of corrosion on aluminum, including how it affects the material's properties and performance.
- <p>Corrosion on aluminum can lead to a loss of structural integrity, reduced strength, and increased brittleness. It can cause pitting, which is localized corrosion that leads to the formation of small holes or cavities on the surface. Over time, this can lead to material failure. Corrosion can also alter the appearance of aluminum, causing discoloration and a rough texture. In extreme cases, it can lead to the complete degradation of the aluminum, resulting in the loss of the material's functionality. Proper maintenance, such as applying protective coatings or using corrosion-resistant aluminum alloys, can mitigate these effects.</p>
- Q: What are the elements that make up aluminum oxide and what is its chemical formula?
- <p>Aluminum oxide is composed of aluminum and oxygen. Its chemical formula is Al2O3, indicating that it consists of two aluminum atoms (Al) and three oxygen atoms (O). This compound is a widely used industrial material, known for its high melting point and hardness.</p>
- Q: Describe the chemical reaction that occurs when aluminum reacts with an acid.
- <p>When aluminum reacts with an acid, it undergoes a single displacement reaction. The general reaction can be represented as: 2Al + 6HCl 鈫?2AlCl鈧?+ 3H鈧? In this reaction, aluminum (Al) displaces hydrogen (H) from the hydrochloric acid (HCl), forming aluminum chloride (AlCl鈧? and hydrogen gas (H鈧?. The aluminum atoms lose three electrons each to form Al鲁鈦?ions, while the hydrogen ions (H鈦? from the acid gain electrons to form hydrogen gas. This reaction is exothermic, releasing heat and often producing bubbles of hydrogen gas.</p>
- Q: Are aluminum alloys suitable for use in the aerospace industry?
- <p>Yes, aluminum alloys are extensively used in aerospace applications due to their high strength-to-weight ratio, corrosion resistance, and good fatigue characteristics. They are particularly favored for constructing airframes, wings, and other structural components where weight savings are critical. The alloys are also used in manufacturing engine parts and heat exchangers due to their thermal conductivity and ability to withstand high temperatures. However, the specific alloy selection depends on the particular requirements of the application, such as temperature resistance, strength, and environmental conditions.</p>
Send your message to us
Aluminum Foil for Blister Packs
- Loading Port:
- China Main Port
- Payment Terms:
- TT or LC
- Min Order Qty:
- 1 m.t.
- Supply Capability:
- 7000MT/MONTH m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords



























