Sodium Dichloroisocyanurate SDIC For Water Treament
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 17 m.t.
- Supply Capability:
- 9000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Sodium Dichloroisocyanurate SDIC
Introduction:
SDIC White powder or grain with chlorine odor . It is a strong oxidant and chlorate agent and can dissolved in water easily . Its aqueous solution
assumes weak acidity and the active chlorine in its dry products lose little when it is stored for a long time at the atmospheric temperature .
Specification:
Chemical Name | Sodium Dichloroisocyanurate | |
Molecular Formula: | C3O3N3HCL2NA | |
Molecular Weight: | 220.96 | |
CAS Number: | 2893-78-9 | |
Product | 60% | 56% |
Available chlorine(%,min) | 60 | 56 |
Moisture content(% max) | 5 | 8 |
PH Value(1% solution) | 6-7 | 6-7 |
Particles Size:
Mesh | 5~8 | 8~30 | 20~40 | 20~60 |

Main usage:
this products can effectively kill various germs, fung uses and viruses, specially A&B type hepatitis viruses. It is effective on killing algae,
decolorizing cleaning water or bleaching .It can be widely used for epidemic prevention, livestock farming , industry and agriculture.

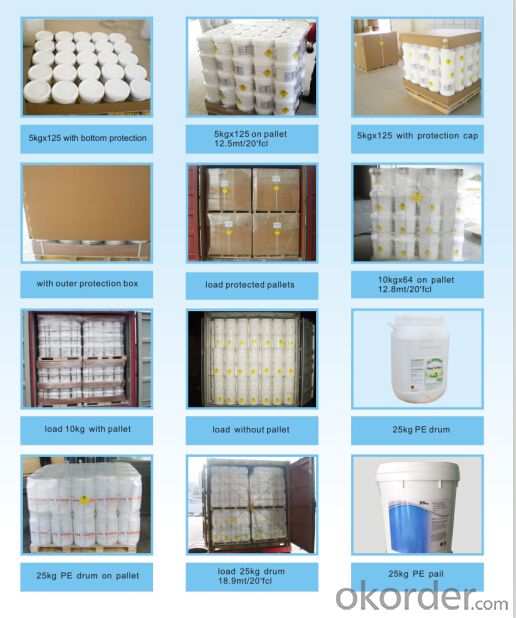
Package:
50KG PLASTIC DRUMS/ FIBER DRUMS.25KG PLASTIC DRUMS/FIBER DRUMS. 1000KG BIG BAGS. Or any other packages
suggest by customers.

- Q: Is the catalyst considered a chemical reaction?
- The catalyst is involved in the reaction, but in the reaction process is a step in the effect of its shape has not changed, so that did not participate in the reaction.The role of the catalyst is to reduce the activation energy of the reaction, the original one reaction into two or Multiple reactions, each sub-reaction of the activation energy is very low, the reaction is very good, the effect is the total reaction faster. Hope to adopt
- Q: What is a catalyst in a chemical reaction?
- A catalyst increases the rate of a reaction by lowering the activation energy. Activation energy is the energy required to make the reactants form the products. If the activation energy is lower the reaction takes place faster and more easily. Also catalysts are not used up during the reaction.
- Q: Chemical equation if there is a catalyst and heating, which write in the equal sign above, which written in the following? Tomorrow academic level test, solution
- At the same time, the catalyst is heated
- Q: how does the amount of a catalyst affect reaction rate?
- theoretically, the more catalyst there is, the faster the rate of reaction. this is because it is bringing more particles together quicker.
- Q: High school stage which organic chemical reactions do not use catalyst
- Olefins, alkynes, making bromine water, potassium permanganate fade.
- Q: Why extract the genome, the digestion is always not cut
- The enzyme, like the general catalyst, only catalyzes the thermodynamics of the permissible chemical reaction, shortening the time to reach the chemical equilibrium without changing the equilibrium point. The enzyme as a catalyst has no qualitative and quantitative changes before and after the chemical reaction. The mechanism of action of enzymes and general catalysts is to reduce the activation energy of the reaction.
- Q: In the chemical reaction will have to use the catalyst reaction, such as H2O2 === (MnO2) H2O + O2 ↑, then the catalyst in the end to participate in the reaction (that is, the catalyst itself is the reactant) If so, why are some of these substances in the reaction (these substances refer to the catalyst) in the reaction after the quality and nature of the change does not change?
- In the chemical reaction can change the chemical reaction rate of other substances (both can also improve), and its own quality and chemical properties in the chemical reaction before and after the material did not change called catalyst (also known as catalyst)
- Q: The catalyst can change the chemical reaction process, why is it wrong?
- Clear catalyst is to change the reaction rate, and some of the catalyst in the reaction is to speed up the reaction rate, and some reactions in the catalyst is to slow down the reaction rate. The catalyst changes the rate of chemical reaction and can not be said to change the course of the reaction
- Q: What chemical reactions can water do the catalyst?
- Many solid and solid reactions can be converted into reactions between liquids, which speeds up the reaction rate, and perhaps the water here is the catalyst.
Send your message to us
Sodium Dichloroisocyanurate SDIC For Water Treament
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 17 m.t.
- Supply Capability:
- 9000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches































