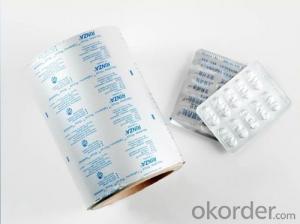
Pharmaceutical Grade Faraday Cage Aluminum Foil
- Loading Port:
- China main port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 3 m.t.
- Supply Capability:
- 5000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Specification
1.Description of Plain Aluminium Foil for Pharmaceutical Use
It's clean,smooth,bright and coating evenly. And stacked,cleft,indentation, oil stain, surface oxidation are not allowed.
2.Why you want to choose us?
We've been specialized in aluminium foil for more than ten years, we know this product very well, we know what is good, what is the market price.
3.Specification and Application of Plain Aluminium Foil for Pharmaceutical Use
1. Alloy 8011 temper h18
2.Thickness 20-30mic
3.Width 50-600mm
4. One side op and other HSL heat seal
a. heat seal 3-4gsm
b. heat seal 4-6gsm
c. heat seal 6-8gsm
5. Colored : gold, sliver
6.Applicaiton : pharmaceutical packing , heat seal against pvc and pvdc ,
7. ID 76mm od 600mm
8. Packing : case in wooden pallet
9. Min order 1ton as trial
10. Produce term 15-20days
4. Property of Plain Aluminium Foil for Pharmaceutical Use
Excellent properties such as of anti-oxygen, moisture-proof, leak-proof, carryhome, antipollution, etc.
High stability and sanitation
Heat-sealing with PVC or PVDC
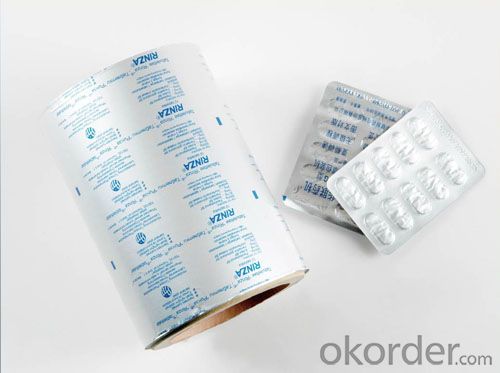
5.Pictures:
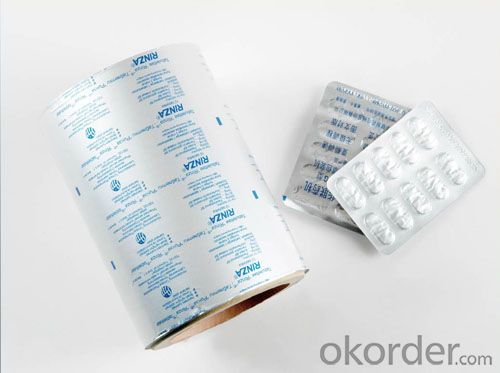

6.FAQ:
1) What is your delivery time?
Our regular production time is over 30 days, It depends on the order quantity also.
2) What is your payment term?
We accept T/T, LC at sight, Usance LC 30, 60, 90, 120, 180 DAYS.
3) What is your price structure?
Our foil price is based on Shanghai Metal Price(SMM), not LME, but we could offer LME+ Conversion for your reference.
4) What is your Delivery term?
We do FOB, CFR, CIF, we don't do DDP.
5) Could you offer sample?
We could offer sample as your requirement. A4 Size sample is free for you, for bigger roll sample, it depends on the coil weight.
- Q: Is it safe to reuse aluminum foil containers that have been used for food storage?
- <p>While it is possible to reuse aluminum foil containers for storing food, it is not generally recommended. Reused aluminum foil can become thin and weak, potentially leading to punctures or tears. It may also lose its ability to seal properly, which could compromise the freshness and safety of the food. Additionally, repeated use can lead to the transfer of bacteria or other contaminants. It's safer to use clean, new aluminum foil each time for food storage to ensure the integrity of the container and the food's quality.</p>
- Q: what's a better more eco-friendly alternative instead of plastic sandwich bags?
- It can unravel but hopefully in this case it won'tIf you don't want to start over there is nothing you can do.
- Q: I've only owned and driven a 69 F-100 Ranger and a 70 F-100The title on the 70,only say's F-100.The dash is painted orangeShould I assume that means it's an ExplorerAnd the question about the HEADLIGHT'S is : What year did they change,from round,to rectanlgeTHANK'S !!!
- After making the shape as suggested, place the 'boat' in a shallow bowl, arrange the pennies evenly on the base and slowly fill the bowl with waterThe boat should rise up evenly and float for ever.
- Q: 28.Based on their positions in the periodic table predict which atom from each of the following pairs has a larger ionization energy:Rb / F Be / Ba Ca / Fe Al / Mga) F, Be, Fe, Al c) F, Be, Fe, Mgb) Rb, Ba, Ca, Mg d) Rb, Ba, Ca, AlMy answer is A, but the right answer is CI don't understand why is Mg and not Al when Aluminum is closer to the noble gases and FluorinePlease explain to me why the answer is what it is.
- Use heat protection products, only straighten it when you need to and condition like there's no tommorowDo that and you should be fine but everyone has different hair but if you notice it getting badly damaged STOP
- Q: It has to be written kinda like thisns2(n-1)d6except correctlol
- Wow I can't believe people are actually telling you thisDon't ever cut I know it gets really hard and trust me I understand I used to do it but it's not worth it babeYour worth so much more than words or any problem going on you should wake up in the morning and tell your self your worth it and you start getting used toBecause trust me I don't need to see a picture of you to tell your beautiful everyone has their own demons and this is one tricky one but fight it fight the urge and grow as a person higher your self esteem and live life the way you want to live it.
- Q: It would appear that a chemical reaction with the aluminum caused a quot;filmquot; to cover the glass since the adjoining window is quot;filmquot; free.If you encontered a similar situation; please advise.
- No I have not try washing the window again , with warm water , vinegar and news paperwear gloves so the dye from the news`paper won?t stain your hand?sThis might help you out, Soak the news paper in the water squeeze some water out and wipe.No streaks
- Q: I have a natural gas dryerIs it safe to use flexible aluminum foil ducting rated to 250F?Thanks
- Just as stated by most people before me, your horse should be fineI would just monitor her and see how she is fairing while out thereShe sounds pretty fit for a 20 year old if you're riding her every day and will probably be just fineThe cows will help keep her from looking as tasty as she might look as a lonerAnd I have seen horses hang around cows quite a bit (it's actually kind of helpful, because then the horse isn't scared of them, and you could use her to help you work the cows - if you wanted that is)Horses and cows are herd animals and like to stick together, so long as one of their herd-mates doesn't want to eat themSince your horse is so close to your house, I wouldn't worry about any coyotesOnly if they are really hungry will they hunt something so largeAnd they do hunt in packs, usually trading off who runs down the prey, thus out-running and tiring out the preyBut like I said before, a horse is a bit big for the little coyotes to go chasing off afterGood luck with your new pony and I would get a lean-to put up as soon as you can, only so that she has the option to use it should she need itHave fun and enjoy her!
- Q: No special chemicals.
- Line a pan with aluminum foilIt can be scrap aluminum, as long as it is cleanPlace your silver on the aluminumBoil water, dissolve a teaspoon of salt and a cup of baking soda into itPour into the pan, covering your silverThe tarnish, caused by sulfur attaching itself to the silver, is transferred to the aluminum via the 'salt bridge', resulting in nice clean silver once again without the hassle of having to polish it.
- Q: I have a house in a major US City with a 3rd-floor outdoor deck that also allows access to the roofThese areas provide my only outdoor areas and excellent views of the city skyline, and thus are ideal for entertainingThe deck is small (about 10' x10'), has an outdoor light, and has one outletThe roof has no electrical outlets or lightingI am trying to find ways, preferably permanent, to light and provide music to these spacesAlso I am a college student on a very tight budget so the cheaper the betterAny and all suggestions are appreciated.
- Dude, Buy a whole bunch (bags) of these little white candlesEach is round, has it's own aluminum baseWe do this with our terrace.you can line then along edges of the balconey, ledges etc Very Euro trendy and cheapAs for music.just get a smal techno bro boxYou don't need to blast loudly .
Send your message to us
Pharmaceutical Grade Faraday Cage Aluminum Foil
- Loading Port:
- China main port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 3 m.t.
- Supply Capability:
- 5000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords