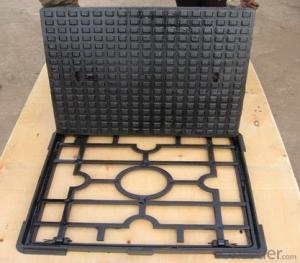
Manhole Cover EN124 E600 Ductile Cast Iron Anti Theft
- Loading Port:
- China main port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 200 pc
- Supply Capability:
- 10000 pc/month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
1,Cast Iron Manhole Cover Description :
A manhole cover is a removable plate forming the lid over the opening of a manhole, to prevent anyone or anything from falling in, and to keep out unauthorized persons and material.
Manhole covers are often made out of cast iron, concrete or a combination of the two. This makes them inexpensive, strong, and heavy, usually weighing more than 50 kilograms (110 lb). The weight helps to keep them in place when traffic passes over them, and makes it difficult for unauthorised people not having suitable tools to remove them.
A manhole cover sits on metal base, with a smaller inset rim which fits the cover. The base and cover are sometimes called "castings", because they are usually made by a casting process, typically sand-casting techniques.
2,Main Features of the Ductile Iron Manhole Cover:
·High endurance
·High Strength
·Pressure Resistence
·Anti-corrosion
·Anti-theft
·Good visual effect
3,Manhole Cover Images:


4,Manhole Cover Specifications:
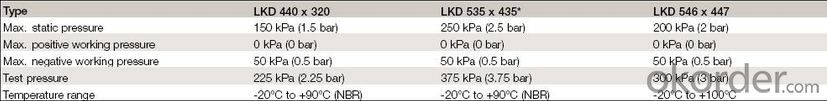
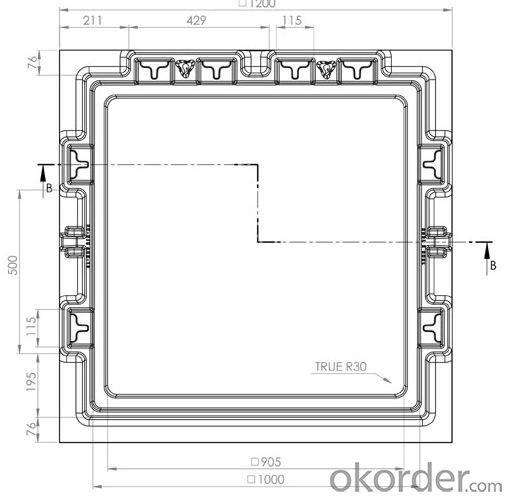
5,FAQ:
We have organized several common questions for our clients,may help you sincerely:
Q. Can I fit a square manhole cover into a round chamber?
A. Yes, there are a select few manhole covers which are square-to-round, meaning they will fit square or round chambers.
Q. I have a heavy traffic manhole cover but it has been broken by a forklift, why?
A. Forklifts have solid wheels which apply pressure differently to standard pneumatics and therefore covers must be specially adapted to suit this.
Q. I’ve measured my clear opening size but none of your covers match it, can you offer anything?
A. It’s likely that your cover is quite old and in imperial measurements which are no longer manufactured. However, we can offer bespoke solutions with manhole covers made to the specification you require.
Q. The project I’m working on requires a manhole cover with a quality finish, what’s available?
A. Naturally, galvanised steel of black polypropylene aren’t always ideal, and so we offer brass or stainless steel edging, as well as complete stainless steel covers.
Q. Odours are coming through where I have installed my manhole cover, why?
A. Your cover must be sealed and locked, or neoprene sealed, so you would require a T-Range Cover, or if you require a solid top, a selection of the PC range (e.g. PC2BG3, PC6CG3, not PC2BG) are suitable.
Q. I need access through my manhole cover on a regular basis, is this possible?
A. Yes, the majority of covers have built-in lifting keys, and for those that don’t we also supply lifting keys. However, the best way to obtain easy access is to have a bespoke, hinged manhole cover.
- Q: what are the best melodic metal bands and or metal bands? thanks!
- its not a redone version..... its the version the ACTUALLY follows the manga... and the manga is done, so there shouldnt be anything trhat follows FMA: Brotherhood
- Q: Water is formed when hydrogen is passed over the heated oxide of metal xNo water is formed when hydrogen is passed over the heated oxide of metal yWhat is the order of reactivity of hydrogen, metal x and metal y?-------Does anyone know the answer and can you please explain why because I don't really understand how this is supposed to get the order of reactivity.
- Volume of metal = total volume,final - initial volume of water. = 23.4L - 15mL = 8.4mL density,metal = mass,metal / volume,metal =8.27g/8.4mL = 0.9845 =0.985g/mL---->ans!
- Q: When metal reacts with water a __________ is produced
- Silver metal is added to hot water or exposed to steam No reaction Zinc metal is added to hot water or exposed to steam Yes produces zinc oxide and hydrogen Chromium metal is added to hydrochloric acid Yes, produces chromium chloride and hydrogen Lead metal is added to cold water No reaction Mercury metal is added to hydrochloric acid No reaction
- Q: Don't say Nu Metal, or Glam, because there are worse genres.
- Furnaces are made out of stone, not metal. Some metals do not need refining. gold, silver can all be found lying on the ground in their metallic state. Metals like copper and zinc can be refined with just a small amount of heat. And iron refining can be done without any metal at all. .
- Q: Apart from the facts that metals always loose electrons and are good electric conductors and that non-metals always gain electrons and are bad conductors, what is the difference between the two?
- Metal is the same temperature as the rest of the room. It just feels cold because it conducts heat so well. When you touch something non-metallic, the surface instantly heats to the temperature of your finger and does not feel cold.
- Q: Hey i would like some help becuase I just really got into metal 1 year ago and I would like some help.I don't want to find some metal heads and act retarded in front of them or act like a poserAnd what to watch out for thx (:
- What is the formula of the metal oxide. MO? M2O? MO2? You need to know in order to solve the problem. I will show you how to solve it, assuming it is MO (if it is not, you will need to account for that). If we pick an arbitrary number, like 100g, 67.2 g is metal and 32.8 g is oxygen. Since the atomic weight of oxygen is 16.0 g, 32.8 g O / 16.0 g/mol = 2.05 moles of oxygen. This is the part where the stoichiometry is important. Depending on the formula, this would determine the number of moles of metal. MO gives you 2.05 moles M (1:1 M:O), M2O gives you 4.10 (2:1 M:O), MO2 gives you 1.025 (1:2 M:O), etc. 67.2 g M / # moles M = MW of M Then use the atomic mass values on the periodic table to figure out what metal it is. Good luck.
- Q: Listen to these metal songs by Korn and try telling me they are not metal:Korn - ClownKorn - Shoots and LaddersKorn - Ball TongueKorn - *****Korn - Blind
- The traditional definition focuses on the bulk properties of metals. They tend to be lustrous, ductile, malleable, and good conductors of electricity. A modern definition of metals is that they have overlapping conduction bands and valence bands in their electronic structure.
- Q: This is my Physics homework and I'm stuck because I was ill for a few lessons.Whole question: ''Compare the similarities and differences between the process of conduction in metals and non-metals.''
- Metals will weaken acids so the pH will get higher. e.g. dilute HCl pH =1 add metal e.g. magnesium pH MgCl2 solution = 7
- Q: Is it possible to react a metal with hydrogen? What would this be called ( for example would cu and h react to form copper hydryde?). I have observed in electolysis cells that the hydrogen metal (if it is not a good anode) turns black. Is this the chlorine frome the salt changing the andode? The other connection turns red from rust. Would this be possible, because hydrogen requires one electron to fulfill its energy level (2) and metals (such as copper) are looking to lose electrons? If not, why?
- Well, we happen to know that the metal in Drano is aluminum. To identify the metal, I would isolate the pieces of metal and measure the density. Most people think that the aluminum reacts with the NaOH. Actually the NaOH dissolves the oxide coating that covers the aluminum metal. Once the Al2O3 is dissolved, the aluminum metal reacts with the water to make aluminum hydroxide and hydrogen gas. Al2O3S) + 6NaOH --> 6Na+ + 2AlO3- This exposes aluminum metal which reacts with water: 2Al + 6HOH --> 2Al(OH)3(s) + 3H2(g)
- Q: Can anybody name about three corrosive metals and three non-corrosive metals? Also is acid corrosive?
- A transition metal carbene complex is a organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. All transition metals form such complexes. Many methods for synthesizing them and reactions utilizing them have been reported. The term carbene ligand is a formalism since many are not derived from carbenes and almost none exhibit the reactivity characteristic of carbenes. Described often as M=CR2, they represent a class of organic ligands intermediate between alkyls (-CR3) and carbynes (≡CR). They feature in many catalytic reactions in the petrochemical industry and are of increasing interest in fine chemicals. The characterization of (CO)5Cr(COCH3(Ph)) in the 1960's is often cited as the starting point of the area,[1] although carbenoid ligands had been previously implicated.
Send your message to us
Manhole Cover EN124 E600 Ductile Cast Iron Anti Theft
- Loading Port:
- China main port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 200 pc
- Supply Capability:
- 10000 pc/month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords