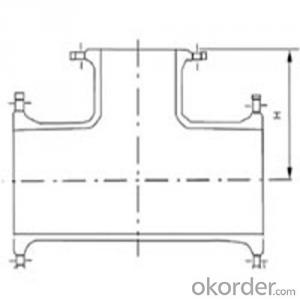
Ductile iron pipe fittings
- Loading Port:
- China Main Port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Specifications
1.ductile iron pipe fittings
2.Quality Management System:ISO9001
3.standard:ISO2531,EN545,BS4772
4.use:connect iron pipe
| material | iron GGG500-7, GGG450-10 |
| standard | ISO2531,EN545,BS4772 |
| size | DN60MM-2000MM |
| market | Europe,Middle east, Africa, America |
| price | FOB |
| delivery | 30 days |
| hardness | maximum≤250HB |
| proof stress | minimum≥300MPA |
| tensile strength | minimum≥420MPA |
| elongation | minimum≥5% |
| payment | T/T |

1. Coating:
fusion bonded epoxy coating;
cement lining inside and zinc and bitumen coating outside;
zinc+bitumen inside and outside
2. Accessories: gaskets,bolts and nuts
3. Minimum order: according to detailed sizes
4. Sample: sample for free, but you should pay for the freight charge for the samples.
5. Delivery date: within 20-30 days after receipt of the 30% payment in advance for one twenty-feet container;
or according to detailed size and quantity
6. Packing methods: wooden case/pallet with plastic layer,or as per customer's requirements,
packing size: according to the products size and quantity.
- Q: Looking for a waffle iron that is truly NON-STICK
- no downside except the brakes may not work right - as the other guy said wle
- Q: i keep having these like dizzy spell where the whole room gets really light and i start falling over but im able to keep myself up. it lasts like 3 minutes at the most. my neighbor told me that i might be amnemic so my mom is taking me to a doctor soon. what can i do before i get a chance to go to the doctor to get more iron. im just scared that its going to happen to me at school.
- The iron in red meat is readily absorbed so it is a good source of iron. Vitamin C also helps to absorb iron so plenty of fruit and vegetables in conjunction with red meat wil boost your iron levels. There is also plenty of iron in nuts.
- Q: i say Spider-Man. He could just pull out Iron Man‘s heart with his webbing.
- Peter Parker the amazing Spider-Man vs iron man that's debatable because he webbed up iron man in amazing spider man 544 (but in a what if he hacked his suit). He also got beat up by iron man in a different issue. During the dying wish storyline Dr octopus and spider-man switched minds and Peter Parker dying inspired dr octopus (or SPock if you will) to become a hero the superior spider man He has claws, spider-bots, a backpack with mechanical arms, spider walkers and an army of spiderlings. Earlier dr octopus beat iron man by hacking his suit so SPock wins. By the way the arc reactor is an electro magnet in the comics it's called a RT node
- Q: How do you prevent iron-on transfers from peeling off fabric?
- If you did the transfer, you need to make sure that your iron is hot enough to adhere the transfer to the fabric. If it begins to peel off, there are commercial adheasives you can use to glue it back on. You can check out your local craft or fabric store for those. After you have applied your transfer, the best way to protect it is by handwashing while the item is turned inside-out. Also, do not use the dryer as this well fade and crack your transfer once your garment is washed.
- Q: how iron work? function of iron ,
- Iron is a key component of hemoglobin and myoglobin which transport oxygen throughout the body. Hemoglobin is found in red blood cells, and myoglobin is found in muscle cells. A deficiency in iron can lead to iron deficiency anemia which means oxygen is not being transported throughout the body efficiently. This leads to fatigue, compromised immune function, and lowers energy metabolism. Iron toxicity is also a concern which leads to nausea, vomiting, stomach irritation, and impaired absorption of other minerals.
- Q: Iron deficiency ANEMIA. What foods to eat to get 18mg of iron a day?
- Well you can always take iron supplements but they will constipate you. Best take iron rich foods and take VITAMIN C with them too as it helps you absorb iron. Here is a list of some Iron rich foods: Foods that are a good source of iron include: (liver, lean red meats, including beef, pork, lamb--just so others know too ) seafood, such as oysters, clams, tuna, salmon, and shrimp, etc. beans, including kidney, lima, navy, black, pinto, soy beans, and lentils iron fortified whole grains, including cereals, breads, rice, and pasta greens, including collard greens, kale, mustard greens, spinach, and turnip greens (the darker the green the more iron) tofu vegetables, including broccoli, swiss chard, asparagus, parsley, watercress, brussel sprouts chicken and turkey blackstrap molasses nuts egg yolks dried fruits, such as raisins, prunes, dates and apricots (and probably PURE juices from these are rich too)
- Q: what are the commonly available iron containing food
- Animal Foods Rich in Iron Eggs - especially the yolk Oily fish e.g. tuna, sardines, pilchards, cockles and mussels Kidney, liver, heart Lamb, game, beef Black pudding, corned beef Oxo, Bovril Vegetable Foods Rich in Iron Wholemeal bread and flour Iron-fortified cereals - Bran, Branflakes, Weetabix Beans and pulses e.g. lentils, chick peas, haricot, kidney, pinto, baked, butter, peas Nuts - almonds, cashews, Brazils, walnuts Dried fruit - apricots, figs, dates Green vegetables - watercress, spinach Rich fruit cake, gingerbread, ginger biscuits Curry powder Cocoa, chocolate, black treacle Have vitamin C with your meal to increase absorption so have a glass of orange juice.
Send your message to us
Ductile iron pipe fittings
- Loading Port:
- China Main Port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords