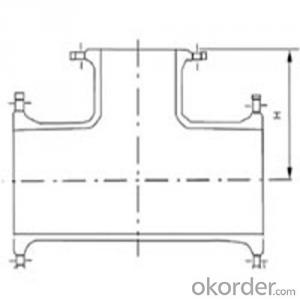
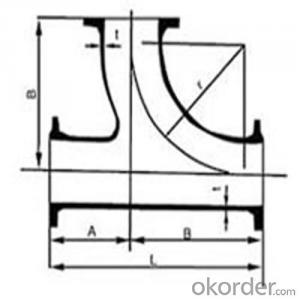
Ductile Iron Bends ISO-2531
- Loading Port:
- China Main Port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Specifications
Ductile Iron Fitting,Class K12,K14
Suppply Potable/Sewage Water
Standard:ISO2531/EN545/EN598
Competitive Price & Good Service
Ductile iron fitting
Key Specifications:
Material : Ductile Cast Iron
Tee/Cross/Bends/Taper/Collar/Cap/Flange Socket/Flange Pipes with Puddle/Duck foot Bend/Flange Bell Mouth/
Size Range : DN 50mm to DN 2600mm
Joint Type : Flange PN10,16,25Bar/ Socket/K Type with Mechanical Gland
Manufacture Standard: ISO 2531/ EN545/EN598/AWWA C110 and C153
Casting Method: Lost foam technique
Annual capacity : 10,000 tons
Coating Exterior: zinc 130g/m2 and bitumen coating 70 microns.
Cement Lining Interior: Portland Cement/ High Alumina Cement/ Sulphate Resisting Cement.
Special requirements on external coating and internal lining can be applied
We also provide accessories such as SBR/EPDM rubber gaskets, bolts&nuts.
Additional Parts:
Each fitting is strictly inspected according to ISO standard to ensure permanently high performance.
Easy Installation at site and service free for life
Long Service Lifespan
Quotation will arrive you within 24hours once we get your inquiry.
We guarantee offering a competitive price to you.
A copy of original inspection reports of pipes will be offered after shipment.
Photos of loading process will be sent to the customer after shipment effect.
We will follow-up the delivery progress after shipment effect and update to the customer on weekly basis
- Q: Twice already I burnt a hole trough my silk dresses. People say to iron silk you need to do it in low heat inside out.
- I always iron inside out any garment. It comes out nicer. Silk is one of the most durable fabrics. I would use low heat, and if you are apprehensive, put a cotton towel between the iron and the fabric.
- Q: How many kinds of elbow of ductile iron pipe?
- There are 12.5 degrees and 22.5 degrees elbow, but manufacturers rarely produce. You can consult the manufacturer on the Internet
- Q: iron i believe come from meteor that enter our system.
- What is natural? If you mean, was it a part of the first ball of elements that congealed into the molten earth, then maybe. There was probably iron in the universe by the time the earth started forming 5 billion years ago, since stars had been expoding out heavy elements for billions of years. But it very likely that the earth was bombared by a LOT of materials that contained iron, water, and other elements as well.
- Q: On the wheeloaders (payloaders) we build at the plant I work at, one of the main lift arms has the words ‘ductile iron, do not weld‘, embossed on it.Oddly enough one of the engineers at the plant could not answer my question. What is ‘ductile iron‘ and why can it not be welded?
- It was like snow in reverse, dry not wet, hot not cold, signifying death not life.
- Q: In my experiment, I ignited the iron before placing it in a jar containing oxygen. The iron had a reaction with the oxygen and there was no more iron left. What happened? Could you add more detail to the outcome of this reaction?
- Iron and oxygen produce rust or Iron oxide when they are joined. However, it is possible that you burned the iron, as oxygen is highly flammable.
- Q: I want to know how long will it take to get back up? I‘m taking 2 Iron tablets a day +s my Prenatal.What kind of food can help too?I appreciate it!,Thank yuHappy Valentines Daylt;3
- Beef is great source of iron, I ate it regularly during my pregnancy because my iron level was always very low (however in range). Remember to eat foods rich in vitamin C at the same sittings as iron-rich foods as it will increase the absorption of iron. The other iron-rich foods are: sardines, baked potatoes with skin, spinach, kale, pumpkin seeds, beans and dried fruits. Also, to enhance the absorption of the iron in the suppplement, take it between meals with frut juice rich in vitamin C. Caffeinated beverages or high-calcium foods can interfere with iron absorption.
- Q: i have a new fish tank and i read something about iron so i was wondering is iron important for fishlike why do fish or its habitat need iron??
- Just to reiterate what Ian has stated, don't worry about iron. A quality fish food will provide all they need. The only time you need to worry about iron is if you have a heavily planted aquarium under moderate to high lighting. And even then, it's not a fish health issue, it's a plant health issue. Too much iron in your water can also be toxic to fish, invertebrates, and plants. There's an interesting relationship between heavy metal toxicity and water alkalinity. Which probably doesn't mean a whole lot to you, but it's another reason to not worry about adding iron.
Send your message to us
Ductile Iron Bends ISO-2531
- Loading Port:
- China Main Port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords