Nodulizer SiMg China Silicon Magnesium
- Loading Port:
- China Main Port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
OKorder Financial Service
You Might Also Like


Product Description
Ferro Silicon Magnesium alloy is one of the most important inputs in the manufacturing process of spheroidal graphite iron and find usage in low carbon grades of steel due to their low carbon content. Rare-Earth-Si-Mg alloy is a configuration of alloy that put RE, Mg, Ca in ferrosilicon, it is also called Magnesium alloy nodulizer. Rare-Earth-Mg-Si is a kind of good nodulizer. It has a big mechanical strength, the effect of deoxidization and desulfurization is strong.
Packaging & Delivery
| Packaging Detail: | 1.Conventional packing ton bag (1,000kg per bag), or small bag inside ton bags(20,25,50 kg per bag). 2.Special product can use moistureproof bags or barel moistureproof bags. 3.It also can be arranged according to the customer request. In woven plastic big bag per MT or as per customers' requirement. |
| Delivery Detail: | 20-30 days after confirming the order |
Specifications
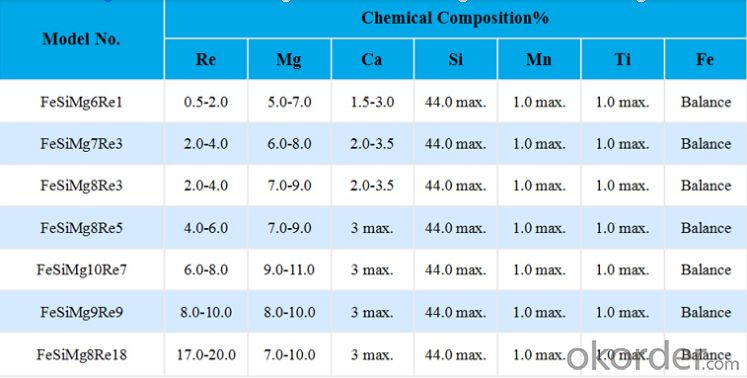
Nodulizer/SiMg/China Silicon Magnesium
1.Original manufacturer and trader
2.SGS/ ICQ/ ISO Approved
- Q: Is it a sub-genre of black metal? Is it basically black metal with pagan lyrics?
- Well I'm really into the Scandinavian Christian Extreme Metal scene, pretty big on Norwegian Black Metal, and also into Finnish Folk Metal.
- Q: Which type of metal is better?
- ...why exactly did you choose baby oil? nevermind, you know there are many many solutions produced solely to bring out the luster of metal? Brasso, Tarn-X, Chromax, etcetra? I'd better suggest you use metal polishing liquids to polish metals.
- Q: are metals Cations or Anions?
- Doom okorder
- Q: hi . element X melts at a temperature lower than that of boiling water. it fails to react with hydrochloric acid and 1centimeter cube (1 cm^3) of the element has a mass of 1.8g. identify element X as a metal or a non metal. Give reasons to justify your answer. thank you
- ALKALI metals: - 1 valence electron, so highly reactive - soft metals - silvery in color - low boiling and melting points - large atomic radi ALKALINE metals: - have 2 valence electrons, still very reactive - shiny, silvery-white colour - when heated in flame, Mg gives a brilliant white flame, Ca a brick-red, Sr a crimson, and Ba an apple green - harder and denser than sodium and potassium, and have higher melting points - found combined with oxygen and other non metals in earth's crust TRANSITION metals: - are electric conductors - have luster - malleable - most are hard solids with relatively high melting and boiling points - because of unpaired d electrons, they can have oxidation numbers of +4, +5, etc.
- Q: and bands mixed with power metal and melodic death metal
- Try listening to Demon Hunter,Underoath,Norma Jean,and Haste The Day.
- Q: I know it'll find anything shiny, but which is the best and cheapest metal dector?Please and thanks!
- well i don't know any false metal band's but i know a real one (im a hardcore fan of this one :P) System of a Down. Their best songs are probably Question! and Hypnotize.
- Q: Which one is better?I'll go on a limb and say Psychedelic rock.
- Silverf^ck
- Q: between metals and non metals?
- I'd Say Dio was arguably the biggest influence of the genre. BQ Venom were more thrash than anything. BQ2 Yeah
- Q: metal?wannabe metal?
- You okorder
- Q: I was having a discussion with my friend about this, I told him that, in my opinion, Glam Metal is a little bit more real than Nu Metal because at least they have guitar solos and at least they have that Metal kind of image, while in Nu Metal, they have no solos and they dressed up as rappers, well some, but they replaced the guitar solos with some Hip Hop influences like DJs and Hip Hop beats and they have a lot of rapping thrown out in there. However, my friend tells me that Nu Metal is more real than Glam Metal because at least, Nu Metal is angry and then he told me that, Metal is always angry. However, in my opinion, I think that Nu Metal is more fake than Glam Metal. What do you think?Which is more fake? Nu MetalNo guitar solosHip Hop influences of DJs and BeatsRappingGlam MetalSlow love BalladsDancing kind of musicWhich one is more fake?
- Death Metal: Minimum break-downs, not many people mosh to it, and you can understand half of it. i.e- Suicide Silence. Black Metal: Shrieking voice, really fast beats, and usually 3 guitarists. i.e-Cannibal Corpse. Thrash Metal: Extremely fast, one of the fastest metals. i.e-Slayer. Hope that is what you were looking for? <3Brecca
Send your message to us
Nodulizer SiMg China Silicon Magnesium
- Loading Port:
- China Main Port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords





















