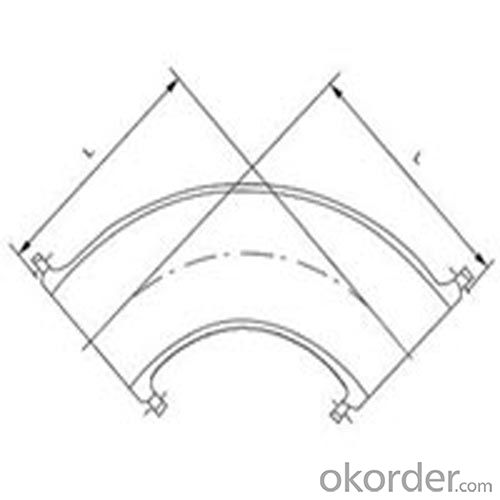
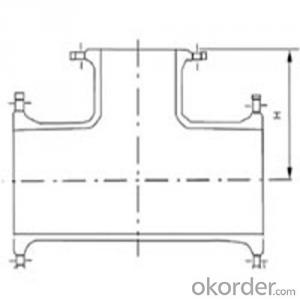
Ductile Pipe Fitting of Double Flange Bend
- Loading Port:
- China main port
- Payment Terms:
- TT or LC
- Min Order Qty:
- 20 m.t.
- Supply Capability:
- 100000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
1.Ductile Iron Pipe Fittings Description :
1) Pipe fittings confirm to ISO2531,K9 class,T type joint,6m long,with inside cements lining conform to ISO4179, outside Zinc spraying(130g/m2) and bitumen coating(70μm) conform to ISO8179.
2) Pipe fittings ends: Spigot and socket ends, with 100% SBR rubber gaskets accoding to ISO4633
3) we can do third party inspection according to customer's request.
2.Main Features of the Ductile Iron Pipe Fittings:
•High yield strength
•High tensile Strength
•High corrosion resistance
•Pressure Resistence
•Anti-corrosion
1).Quality guarantee
• Chemical checking
• NDE after rough machining
• Mechanical testing after heat treatment
• Final NDE,dimension inspected
2).Quality document
• Full Q.A document as per client request
3).Packing and Shipping
• standard export package(carton/wooden case/pallet)
• accept FOB,FAS,CNF,CIF door to door etc or customer designated shipping agent
3.Ductile Iron Pipe Fittings Images:


4.Ductile Iron Pipe Fittings Specification:
Surface Finishes: Bare, Oiled, Mill Varnish, Galv,FBE, FBE Dual, 3LPE, 3LPP, Coal Tar,Concrete Coating and Tape Wrap
End Finishes: Beveled, Square Cut, Threaded, hat
Additional Services: Internal Coating
Packaging: packed in bag, plastic bag, steel strip, steel wire,double wire, iron box, wooden box, tarpaulin, plastic ,sheeting
Inspection: MOODY SGS BV GL DNV ABS LIOYD’S
Test: X-ray, UT, magnetic particle,inspection,hydrostatic test.
Processing service: Beveling, Threading, Slotting, Cut-to length, Bends, Quench and Temper, Fabrication, Double-jointing and On-site assistance
Internal lining: ductile iron pipe fittings shall have an internal cement mortar lining in acc with ISO4179.
External coating: ductile iron pipe fittings shall be externally coated with metallic zinc spray plus a further layer of resin painting to ISO8179.
Gasket: 100% SBR/NBR/EPDM rubber gasket in accordance with ISO4633.
Packing: ductile iron pipes from DN100 to DN300 be bundled with steel belts, others are in bulk.
Payment term: L/C, T/T.
5.FAQ:
We have organized several common questions for our clients,may help you sincerely:
1.Q: Why would you choose ductile iron pipe fittings rather than other pipe fittings materials?
A:The reasons are obvious for that not only ductile iron pipe fittings possesses the inherent strength and flexibility of ductile iron, combined with proven corrosion protection systems, but also the cost savings can be achieved from design to installation and commissioning.
2.Q:Why can you guarantee the inner of pipes can’t be corroded?
A: High alumina cement mortar lining and sulphate-resistant cement mortar lining. These two special linings are applicable to inner anti-corrosion for sewage pipes fittings, improving resistance to erosion of the sewage components.
- Q: Miura vs Mizuno Irons? Which forged iron feels better? Which iron is better do you think?
- I've played Mizuno mp-57 and mp-60's my entire life. The feel and trajectory of Mizuno irons is second to none
- Q: Black, pink, or precious metals straightening iron?
- This Site Might Help You. RE: is there any additives that work for transmission slipping? is there any transmission fluid additives at the auto parts store that actually work for a slipping transmission?
- Q: why do we need alloys of iron?is it linked to the properties of iron metal?
- iron is a very strong metal.very desirable in industrial application,sa main part of steel.also one of the only metal,s that can be magnetizedpermanently.
- Q: I‘m trying to start reading the original Iron Man Comics. I know the first Iron Man is Tales of Suspense #39. Does anyone know what Iron Man #2, #3 etc. is?
- If you are looking to read Iron Man comics from the beginning you may want to start with ESSENTIAL IRON MAN. ESSENTIALS also referred to as 'phonebook collections' are cheap black and white re-prints of early marvel comics. Each volume contains anywhere from 20 to 30 issues of the comic in it. Iron Man himself was 4 Essential Volumes right now. Volume #1 - Containts Tales of Suspence 39-72 Volume #2 - Contains: Tales of Suspence 73-99 Iron Man 1-11 Volume #3 - Contains: Iron Man 12 - 38 Volume #4 - Contains Iron Man 39 - 61 If you've not read other Iron Man books, it is essentiall you find DEMON IN A BOTTLE and probably ARMOR WARS. Otherwise you can start reading it with Matt Fraction's most recent run INVINCIBLE IRON MAN which has been astounding since it's launch. The first 4 trades of this are already out, the 5th is pending The latest issue just came out and it is the perfect jumping on point
- Q: My iron was 32 on a recent blood test and I looked back and last year at this time it was 146. my hemoglobin and hematocrit are within normal limits and when I got the blood test I had been off my period for about 2 weeks (I have moderate periods that last about 3 days and I only bleed heavy for the first day) should I be concerned? I asked a doctor that I work with and he said because I am a menstuating woman it is ok. I am just worried about the drop from last year to this year. anyone. thanks in advance for your answers.
- Perhaps you need more vitamin C to help absorb more iron. Most nuts contain plenty of iron and liver even more. Red meat is a good source of readily absorbable iron and there is also a reasonable amount of iron in bread, cereals and muesli. Oysters and mussels are also rich in iron. Spinach and broccoli contain some iron as well.
- Q: What brand of hairdryers and flat irons would you recommend?
- specific you need to. I in uncomplicated terms flew very final week and introduced in right this moment forward words shop on and my curling iron plus my makeup bag grew to become in my suitcase. My beginning up is liquid and that i additionally had primer and my mascara. there grew to become additionally no subject with the curling iron and mine is a a million and a million/2 barrel
- Q: Who would win and why?I say Iron Fist!
- Batman because he's better.
- Q: I‘m a teenage girl and I‘m almost positive I have an iron deficiency, or anemia, or whatever.I get tired all the time, I‘m unusually pale, I have trouble walking a flight of stairs (I‘m not obese, my weight is normal for my height), and I can sleep for 10 hours but wake up as tired as I was before.How many milligrams of iron should I take? I heard if you take too many it can lead to death. and should I consult a doctor before doing so?
- Iron deficiency is the most common mineral deficiency in women. The best source of iron is obtained from eating meats (Heme-Iron sources). Vitamin C also helps with absorbing Iron especially if you don't eat much meat and get your iron from other foods (Non-Heme Iron). Then your last option would be an iron supplement, but that is never the most effective to get any vitamins or minerals. Here is website with some good iron sources and more information. You should consult your doctor if don't feel better. www.mckinley.illinois.edu/Handouts/dietary_sources_iron.html
Send your message to us
Ductile Pipe Fitting of Double Flange Bend
- Loading Port:
- China main port
- Payment Terms:
- TT or LC
- Min Order Qty:
- 20 m.t.
- Supply Capability:
- 100000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords