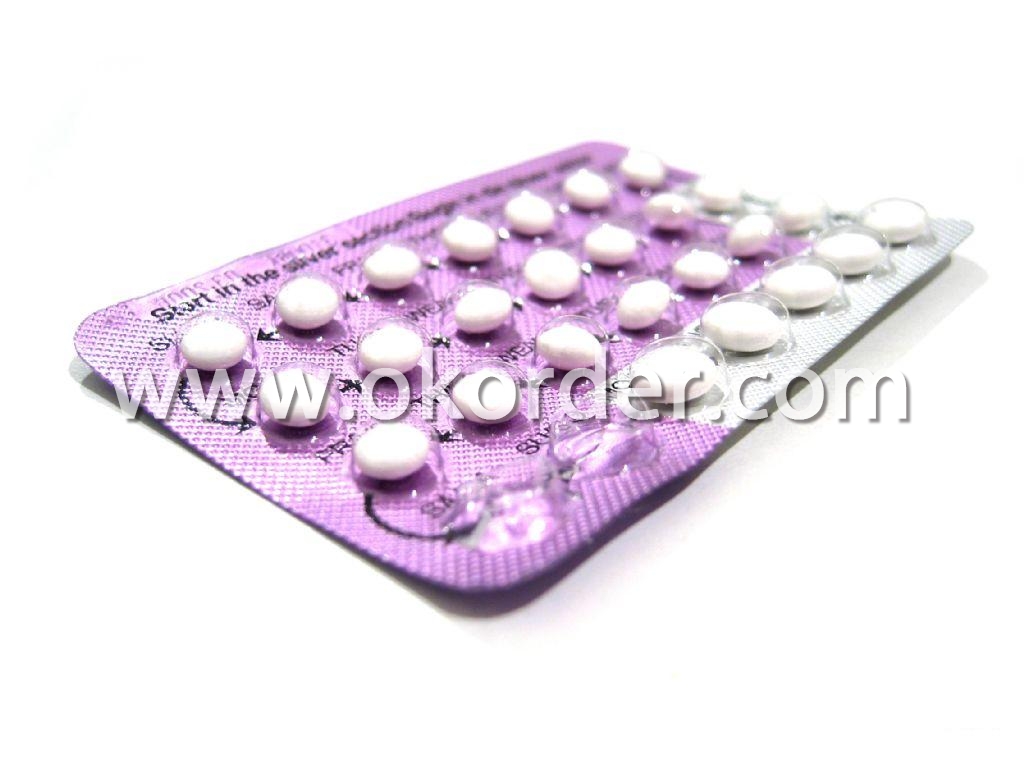
Aluminum Foil for Blister Packs
- Loading Port:
- China Main Port
- Payment Terms:
- TT or LC
- Min Order Qty:
- 1 m.t.
- Supply Capability:
- 7000MT/MONTH m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Quick details of Aluminium Foil For Blister
Alloy: 8011/8006/8079
Temper: H18/O
Specifications of Aluminium Foil For Blister
Thickness & Tolerance: 0.02mm-0.09mm (±6%)
Width & Tolerance: 200 - 1650 mm (+/-1 mm)
Mechancial Properties: Tensile Strength(U.T.S)≥160Mpa, Elongation≥1%
Standard: GB/T3198 / ASTM-B209 / EN546
Usage/Application of Aluminium Foil For Blister
For PTP foil, cord-forming foil, alu-alu foil
Packaging & Delivery of Aluminium Foil For Blister
Packing: seaworthy wooden box with pallet
Delivery: to be loaded by 1 x 20 feet container

- Q: This question asks for methods to determine the presence of aluminum in an alloy.
- <p>To determine if an alloy contains aluminum, one can use various analytical techniques. A common method is spectroscopy, which can identify the presence of specific elements by analyzing the wavelengths of light absorbed or emitted. Another approach is the use of a spark test, where a sample is struck against a grinding wheel to produce sparks; the color and pattern of these sparks can indicate the presence of aluminum. Chemical tests, such as the reaction with sodium hydroxide, can also be used, as aluminum reacts with NaOH to produce hydrogen gas. Lastly, X-ray fluorescence (XRF) is a non-destructive technique that can accurately determine the elemental composition of an alloy, including aluminum content.</p>
- Q: What are the sustainable methods that can be employed in the manufacturing process involving aluminum?
- <p>Yes, sustainable methods can be employed in aluminum manufacturing. These include using recycled aluminum, which requires significantly less energy than mining and refining new aluminum. Implementing energy-efficient technologies and reducing emissions in the production process are also crucial. Additionally, investing in research for new, more sustainable aluminum alloys and manufacturing processes can lead to reduced environmental impact. Lastly, proper waste management and recycling programs can help minimize the environmental footprint of aluminum production.</p>
- Q: Is it safe or permissible to use aluminum in food packaging materials?
- <p>Yes, aluminum is commonly used in food packaging due to its lightweight, durability, and excellent barrier properties against air, moisture, and light. It is used in various forms such as aluminum foil, cans, and flexible packaging materials. However, it's important to ensure that the aluminum packaging is food-grade and complies with safety regulations to prevent any potential health risks associated with aluminum exposure.</p>
- Q: This question asks about the potential health hazards that can result from exposure to aluminum.
- <p>Aluminum exposure has been associated with several health risks, although the scientific consensus is not entirely clear due to varying study results. Some potential health risks include neurological disorders such as Alzheimer's disease, although this link is still debated. Aluminum may also contribute to bone diseases, as it can interfere with calcium absorption. There are concerns about aluminum's role in kidney dysfunction and possible links to certain types of cancer. However, it's important to note that the human body naturally excretes aluminum, and only a small fraction of ingested aluminum is absorbed. More research is needed to establish definitive links between aluminum exposure and health outcomes.</p>
- Q: This question asks for recent applications of aluminum in the transportation industry.
- <p>Aluminum has found new uses in transportation due to its lightweight and high strength properties. It's increasingly used in electric vehicles to reduce weight and extend battery life. Aluminum alloys are also utilized in the construction of high-speed trains and aircraft to improve fuel efficiency and performance. In addition, aluminum is employed in the manufacturing of automotive parts such as engine components, wheels, and body panels. Furthermore, it's used in the production of lightweight bicycles and other transportation infrastructure like bridges and road signs. The trend towards more sustainable and efficient transportation continues to drive innovative uses for aluminum.</p>
- Q: What is the oxide layer that forms on aluminum and what are its properties?
- <p>The oxide layer on aluminum is a thin, transparent, and hard layer of aluminum oxide (Al2O3) that forms naturally when aluminum is exposed to air. This layer is highly resistant to corrosion and protects the underlying aluminum from further oxidation. It is chemically inert, which means it does not react easily with other substances, and it has a high melting point, making it stable at high temperatures. The oxide layer also has a high hardness, which helps to prevent scratching and wear. This protective oxide layer is self-healing, meaning that if it is damaged, it can regenerate and continue to protect the aluminum.</p>
- Q: This question asks about the impact of aluminum on human health, including potential effects and health concerns.
- <p>Aluminum is a common element that can be found in various forms in the environment. While it is not considered an essential nutrient for the human body, it can be ingested through food, water, and medications. In small amounts, aluminum is generally considered safe and is excreted by the body. However, excessive exposure or intake can lead to potential health concerns. Aluminum has been linked to neurological disorders, particularly Alzheimer's disease, although the exact relationship is still a subject of debate. It can also cause bone disorders and affect kidney function. The human body has mechanisms to regulate aluminum levels, but individuals with impaired kidney function may be at a higher risk of aluminum toxicity.</p>
- Q: Is aluminum a suitable material for constructing aerospace structures?
- <p>Yes, aluminum is widely used in aerospace structures due to its high strength-to-weight ratio, corrosion resistance, and ease of fabrication. It is particularly favored for aircraft frames, wings, and fuselage components. Aluminum alloys are also used in spacecraft and satellite structures for their lightweight properties and ability to withstand extreme temperatures.</p>
- Q: This question asks for examples of alloys that include aluminum as one of their components.
- <p>Aluminum is a versatile metal that forms many alloys, enhancing properties like strength, corrosion resistance, and electrical conductivity. Some common aluminum alloys include: 1. 6061, used in aircraft structures and automotive parts due to its good mechanical properties and machinability. 2. 2024, known as 'Alclad', is strong and used in aircraft structures. 3. 7075, one of the strongest aluminum alloys, used in aerospace and military applications. 4. 3003, used for cooking utensils and chemical equipment due to its good corrosion resistance. 5. 5052, used in marine applications and food processing equipment for its excellent corrosion resistance. These alloys are widely used in various industries due to their unique properties.</p>
- Q: What are the methods to decrease the expenses associated with recycling aluminum?
- <p>Yes, there are several ways to reduce the cost of recycling aluminum. One effective method is to improve collection efficiency by consolidating recycling programs and using smart waste management systems. Another approach is to invest in advanced recycling technologies that can sort and process materials more efficiently. Additionally, increasing consumer awareness about the importance of recycling and encouraging participation can lead to higher volumes of recycled materials, which can lower per-unit processing costs. Lastly, government incentives and subsidies can help offset the costs of recycling operations, making it more economically viable.</p>
Send your message to us
Aluminum Foil for Blister Packs
- Loading Port:
- China Main Port
- Payment Terms:
- TT or LC
- Min Order Qty:
- 1 m.t.
- Supply Capability:
- 7000MT/MONTH m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords



























