Coolplas cryolipolysis fat freezen body slimming machine price good
- Loading Port:
- China Main Port
- Payment Terms:
- TT or LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Structure of Coolplas Cryolipolysis Fat Freezen Body Slimming Machine Description
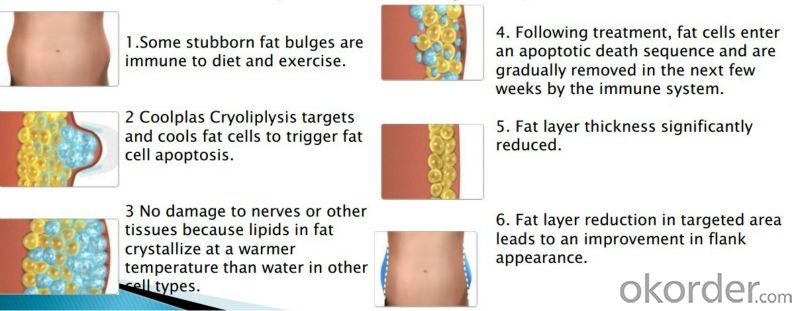
Cryolipolysis treatment is a non-invasive procedure, which uses a targeted cooling process to kill the fat cells underneath the skin, freezing them to the point of elimination. Only fat cells are frozen. Your healthy skin cells remain, well, healthy. No knives. No suction hoses. No needles, No scars. Once crystallized, the fat cells die and are natuarally eliminated from your body.
Main Features of the Coolplas Cryolipolysis Fat Freezen Body Slimming Machine
1. Most popular way in losing weight in the market!
2. Special design for handpeice, no any pain feeling when finished the treatment.
3. No surgery, no anesthesia, no needles, no scarring, no downtime.
4. Single treatment can reduce 20-30% reduction in the fat layer
5. The CoolipoTwin Procedure is comfortable for most patients; They can read, work on their laptop computer, listen to music
6. Automatic adjustment of vacuum pressure control, very little noise at work.
Coolplas Cryolipolysis Fat Freezen Body Slimming Machine Images
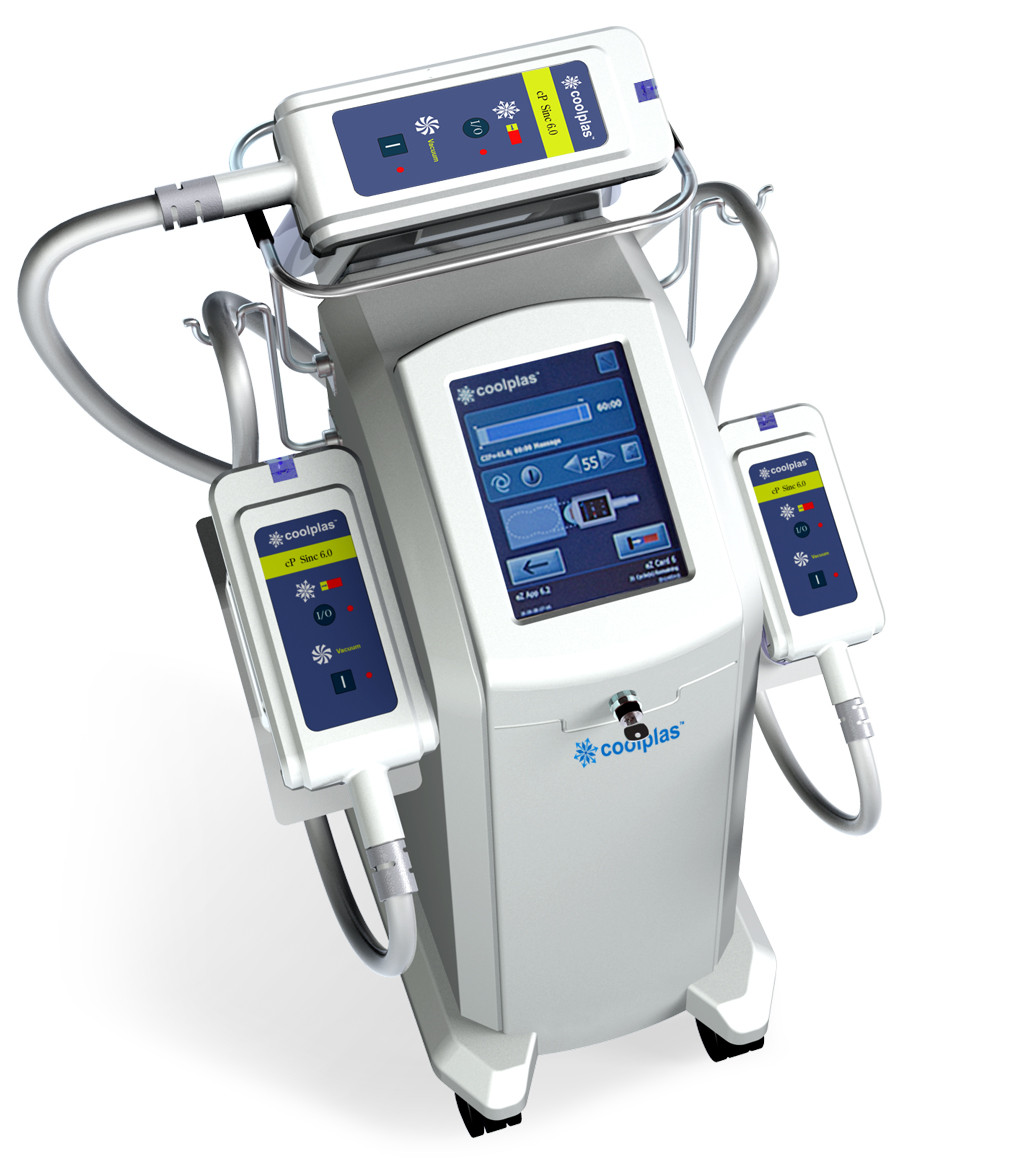

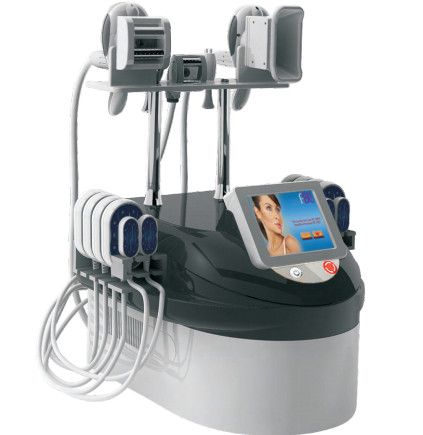
Coolplas Cryolipolysis Fat Specification
Temperature rage | -11℃~0℃ |
Temperature Accuracy | ±0.1℃ |
Treatment area size | Mid: 250mm×50mm Min: 145mm×50mm Fit: 140mm×50mm |
Negative pressure | 0-0.07 M Pa |
Duration | 1~60min |
Electrical requirement | 230VAC 50Hz 1500W |
Dimension(W×D×H) | 300mm×400mm×900mm |
Weight | 30kg |
FAQ of Coolplas Cryolipolysis Fat Freezen Body Slimming Machine
Mid hand: applicator cup dimension 250x50x115mm, effective treatment area:156x86mm
Min hand: applicator cup dimension 145x50x93 mm. effective treatment area:92x66mm
Fit hand: applicator cup dimension 140x50x98mm, effective treatment area 92x66mm
- Q: Private hospital medical equipment procurement
- After receiving the approval, the hospital can carry out the equipment procurement work, and in accordance with the relevant provisions of the "B large medical equipment configuration permit." Private hospitals to implement localized management, all qualified, in line with local health planning requirements, and included in the "second five" during the large-scale medical equipment configuration within the scope of private hospitals can be submitted to the local health bureau filing applications, the results reported to Yunnan Provincial Health Department audit filing, issued access approval, issued "B large medical equipment configuration permit." Private hospitals in the receipt of access to approval, you can carry out equipment procurement work, and in accordance with the relevant provisions of the "B large medical equipment configuration permit."
- Q: What are the main equipment and materials composition of low temperature cold storage? How is the main working principle?
- Suitable insulation layer, refrigeration unit (air-cooled or water-cooled); evaporator (fan or pipe), connecting lines and electronic control system and other components. The principle of professional and strong, you can refer to the working principle of the freezer pondering what is easy to understand.
- Q: Chapter 6 of the management and use of large medical equipment
- The Measures have been implemented since March 1, 2005, and the Interim Measures for the Administration and Application Management of Large Medical Equipment issued by the Ministry of Health Decree No. 43 of 1995 shall be abolished at the same time. December 31, 2004 Large medical equipment management items (first batch) (PET - CT, including positron emission tomography PET) 2, Gamma - ray stereotaxic treatment system (γ - ray emission tomography) (MM50)
- Q: Cryogenic cooling circulating pump can be equipped with what equipment
- The equipment is particularly suitable for the need to maintain low temperature, room temperature work under the chemical, biological, physical laboratories, medical and health, chemical industry, food industry, metallurgical industry, universities, scientific research, genetic engineering, Polymer engineering and other laboratory equipment necessary (according to user needs customized large-capacity low-temperature coolant circulating pump).
- Q: What kind of low temperature drying equipment
- Technical Parameters: 1, temperature and humidity dual control, low temperature and low humidity 2, the compressor cooling 3, electronic temperature and humidity sensor sampling 4, computer intelligent control 5, digital temperature and humidity table 6, the temperature range: 5 ~ 40 ℃ 7, humidity range: 10-40% RH
- Q: Medical disposable surgical kits belong to several types of medical device equipment and numbered codes
- Production conditions: product registration card, production license. Operating conditions: second class operating record certificate.
- Q: Medical low temperature refrigerator offer is how much
- ③ capacity: the greater the capacity, the higher the price.
- Q: Usually high-grade wards are made with a combination of fresco-style equipment and bed locator, as if Wuxi Ningfeng Electronics specializes in this, you can look at their website.
- Seems to be a legal capital, treatment is also quite good. The The The The The Now this kind of foreign-funded enterprises do not seem to recruit through the talent network, do not know what way to understand the recruitment of foreign investment information
- Q: Medical low temperature refrigerator PQ (performance confirmation) has no one done!
- According to the provisions of the medical refrigerator applicable to the national standard,
- Q: What is the medical cleaning device? What should I consider? Solution
- Medical equipment cleaning equipment is currently the international use of ion exchange method, reverse osmosis and ion exchange combination method and reverse osmosis and EDI combination method to prepare medical equipment cleaning water, the water treatment equipment manufacturers aimed at medical cleaning equipment market development space, have Into the R & D industry. According to the different preparation process of ultra-pure water, presumably the development of the device will have their own advantages it.
Send your message to us
Coolplas cryolipolysis fat freezen body slimming machine price good
- Loading Port:
- China Main Port
- Payment Terms:
- TT or LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches