HPMC/HEC for Industrial Construction Wholesale
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5000 kg
- Supply Capability:
- 5000000 kg/month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Building Construction Hydroxypropyl Methyl Cellulose (HPMC)
Brief introduction:
Hydroxypropyl Methyl Cellulose (HPMC) helps building materials apply more easily and perform better. They provide water retention and cohesiveness to mixtures. With special modification, it can be used to control thickening, water demand, workability, sag resistance, strength and other important properties of the final product.
It is widely used as thickener, adhesive, water preserving agent, film-foaming agent in building materials, industrial coatings, synthetic resin, ceramic industry, medicine, food, textile, agricultural, cosmetic and other industries.
Physical and chemical index:
Item | Specification |
CAS NO. | 9004-65-3 |
Appearance | white or light yellow powder |
Moisture Content | ≤5.0% |
PH | 4.0-8.0 |
Particle Size | min. 98% pass through 100 mesh |
Viscosity | 100cps-200000cps, 2% solution |
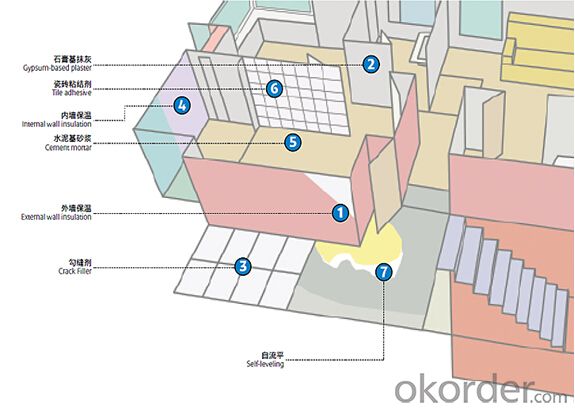
Application in Building:
External wall insulation system (EIFS)
>Bond strength: HPMC can provide the greatest degree of high adhesive bond strength of mortar.
>Performance: The mortar added HPMC has the right consistency, non-sagging. When using, the mortar is easy to work continuously, uninterrupted.
>Water retention: HPMC can wet the wall insulation easily, easy to paste, and also make other additional materials reach the best affects.
>Absorbent: HPMC can minimize the air-entraining volume, lower water absorption of mortar.
>Recommended brand: 75CMAX75000(S), 75CMAX100000(S), 75CMAX200000(S)
Interior and exterior wall interface agent
>Easy to mix, without of agglomeration: HPMC can significantly reduce the friction in the dry powder during the process of mixing with water, which makes it easy to mix and save the blending time.
>Water retention: HPMC can significantly reduce the moisture absorption by the wall. Good water retention can ensure the cement compound with a longer time, also can ensure that workers are able to carry out many times of scraping for the putty on the wall.
>Good working performance stability: even in high temperature environment, HPMC can still maintain good water retention. it is suitable for construction in the summer or hot areas.
>Increased water demand: HPMC can significantly improve the water demand of the putty materials. On the one hand, it improves the operational time after putty put on the wall, on the other hand, it can increase the coating are of the putty, which can make the formula more economical.
>Recommended brand: 75CMAX60000(S), 75CMAX75000(S)
Tile adhesive
>Water retention: HPMC can reduce the moisture absorbed by the substrate and the tile, retain the moisture in the adhesives as much as possible, making mortar still have adhesion after coating for a long time. Significantly extend open time and makes bigger coating area for the worker each time, and improve the efficiency.
>Improve bond strength, improve anti-slip performance: HPMC ensure non sagging of the tiles during working, especially for heavy tile, marble and other stone materials.
>Work performance: The lubricity of HPMC can increase the workability of the mortar significantly, which makes the mortar easy to coating and improve efficiency.
>Improve mortar wetting property: HPMC give mortar consistency, enhance the wetting ability of mortar and substrate, increase the binding strength of wet mortar, especially for the recipe with high water cement ration;
>Recommended brand: 75CMAX40000(S), 75CMAX75000(S), 75CMAX100000(S)
Crack Filler
>Workability: provide the right viscosity, plasticity, and easy to work;
>Water retention: can make the slurry fully hydrated, extending the working time and avoid cracking.
>Anti-hanging: HPMC can make a strong adhesion on the surface for the slurry and not sag;
>Recommended brand: 75CMAX40000(S), 75CMAX75000(S), 75CMAX100000(S)
Self-leveling mortar
>Prevent bleeding: HPMC can play a very good role to prevent the slurry sedimentation, bleeding.
>Maintain liquidity, and improve retention: low viscosity HPMC will not affect the slurry flow effect and easy to work. While possesses certain water retention, makes the good surface effect after self-levelling and avoid cracks.
>Recommended brand: 75CMAX400~600
Gypsum-based plaster
>Water retention: HPMC can retain moisture in the mortar, thus make gypsum completely solid. The higher the viscosity is, the stronger the water-retention capacity, vice versa..
>Sag resistance: allow the worker make the thick coating without causing ripple building.
>Mortar yield: For fixed weight of dry mortar, the exist of HPMC can provide more wet mortar.
>Recommended brand: 75CMAX75000(S), 75CMAX100000(S)

FAQ
Q1.Could we have the sample to test the quality?
Kindly send us your address, we are honored to offer you samples.
Q2. How does your company do regarding quality control?
CNBM a Chinese state-owned enterprise ranked 270th among the global fortune 500 in 2015,
have accreditation in line with standard:ISO 9001:2000,SGS,CIQ certificate.
Q3:What's your Delivery Time?
In generally, the delivery time is 25 days-30 days.We will make the delivery as soon as possible with the guaranted quality.
Q4:What is the convenient way to pay?
L/C , T/T ,Paypal, Western Union and Escrow are accepted,and if you have a better idea , please feel free to share with us .
Q5:Which mode of transport would be better?
In general,we advice to make delivery by sea which is cheap and safe.Also we respect your views of other transportation as well.
- Q:Why the catalyst after the chemical reaction of its quality and chemical properties unchanged
- In fact, the catalyst in the reaction process has become other substances, but after the end of the reaction, the catalyst has changed back. That is, the catalyst is actually involved in the reaction, except that the amount of catalyst being reacted is as much as it did.
- Q:and what type of macromolecule are they made of? thanks!
- by definition a catalyst is a substance that alters the cost of, or makes accessible, a chemical or biochemical reaction yet maintains to be unchanged on the tip of the reaction. Enzymes are the only organic biochemical catalysts. Ribozymes are a particular sort of enzymes. certainly, the definition of enzyme rates: organic and organic catalyst produced in cells, and able to dashing up the chemical reactions mandatory for all times. they're great, complicated proteins, frequently soluble, and are noticeably specific, each and every chemical reaction requiring its very own specific enzyme. The enzyme's specificity arises from its energetic website, a community with a shape such as portion of the molecule with which it reacts (the substrate). the form of the enzyme the place the chemical binds in straightforward terms facilitates the binding of that distinctive chemical, such as a particular key in straightforward terms working a particular lock (the lock and key hypothesis). The enzyme and the substrate slot jointly forming an enzyme–substrate complicated that facilitates the reaction to ensue, and then the enzyme falls away unaltered. In prepare maximum catalysts are used to velocity up reactions. There are different non-organic and organic catalysts. maximum of that are utilized in industry and are commonly transition metals or their compounds.
- Q:The chemical equation of heating reaction of benzene and hydrogen under the action of catalyst
- C6H6 benzene + 3H2 - (arrow) C6H12 cyclohexane (Ni catalytic heating)
- Q:1. Catalysts can help to bring the reactants together in the correct orientation2. The chemical formula of a catalyst is written on the left hand side (reactant) side of an equation.3. Catalysts can provide a surface on which the reaction occurs.4. Catalysts increase the activation energy.5. Catalysts increase the magnitude of the equilibrium constant, thus favoring product formation.6. "Enzymes" are biochemical catalysts.7. Catalysts increase the rate of a reaction.8. Catalysts are slowly used up during the reaction and need to be replaced.
- 1 (I don't know about 2), 3, 5, 6, 8 are true.
- Q:the heterogenous catalyst ZSM-5 IS used to convert ?
- Zeolite-based heterogeneous catalysts are used by industrial chemical companies in the interconversion of hydrocarbons and the alkylation of aromatic compounds. A very good example is the zeolite ZSM-5. This zeolite, developed by Mobil Oil, is an aluminosilicate zeolite with a high silica and low alumininum content. Its structure is based on channels with insecting tunnels. The aluminium sites are very acidic. The substitution of Al3+in place of the tetrahedral Si4+ silca requires the presence of an added postive charge. When this is H+, the acidity of the zeolite is very high. The reaction and catalysis chemistry of the ZSM-5 is due to this acidity. The ZSM-5 zeolite catalyst is used in the petroleum industry for hydrocarbon interconversion. An example use is in the isomerizations of xylene- from meta to para-xylene. The acidic zeolite promotes carbocation isomerizations. There are two suggested mechanisms for this type of isomerizations. Firstly shape may play a role. Perhaps para-xylene has a shape which allows it to diffuse rapidly through the zeolite structure, whereas as meta-xylene takes longer to pass through the zeolite and thus has more opportunity to be converted into the para-xylene. Secondly, is that the orientation of reactive intermediates within the zeolite channels favors specifically para-xylene.
- Q:An important property of the catalyst is that the reaction equilibrium is not changed while increasing the forward reaction rate and the reverse reaction rate. However, because the enzyme for the specificity of the substrate, is not almost every reaction by the enzyme are one way to do it.
- Enzymes, refers to the biocatalytic function of the polymer material, in the enzyme catalytic reaction system, the reactant molecules known as the substrate, the substrate catalyzed by the enzyme into another molecule. Similar to other non-biocatalysts, the enzyme changes the reaction rate by adjusting the Gibbs free energy of the chemical reaction, and most of the enzyme can increase the rate of its catalytic reaction by a million times; in fact, the enzyme is provided with another The activation energy requires a lower route so that more reactive particles produce more effective collisions to produce more kinetic energy. According to the first law of thermodynamics, the kinetic energy obtained by the collision can accelerate the reaction rate by transformation. The enzyme as a catalyst itself is not consumed in the reaction process nor does it affect the chemical equilibrium of the reaction.
- Q:Briefly define a homogenous catalyst? Help please!?
- Define Homogenous
- Q:What is the microcosmic principle of the catalytic reaction in the chemical reaction?
- It is actually directly involved in the reaction, but, after the reaction, it has become a product out, the equivalent of no response
- Q:CO and NO react under the action of a catalyst to generate chemical formulas for CO2 and N2.
- N from +2 to 0 price 2e * 2
- Q:My chemistry teacher wont tell me because it's in the higher course. And i'm not waiting a whole year to find out. And also, google is being a gimp about it. So thanks a lot if you know, I only have basic chemistry knowledge btw, lumen'ss terms if you can.
- Catalysts facilitate the reaction. They might work in several ways. Here is an example: Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process regenerating the catalyst. The following is a typical reaction scheme, where C represents the catalyst, X and Y are reactants, and Z is the product of the reaction of X and Y: X + C → XC (1) Y + XC → XYC (2) XYC → CZ (3) CZ → C + Z (4) Although the catalyst is consumed by reaction 1, it is subsequently produced by reaction 4, so for the overall reaction: X + Y → Z They might also just increase the surface area, thus speeding up the reaction. Example: Coke looses its fizz over time if left with the cork unscrewed. This is because the HCO3 is released as CO2. If you drop a menthos into the coke, it explodes with CO2, because the methos is full of tiny dents in the surface (thus giving it a massive surface area). (i blatantly copied the first example from the wiki)
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
HPMC/HEC for Industrial Construction Wholesale
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5000 kg
- Supply Capability:
- 5000000 kg/month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products
Hot Searches


























