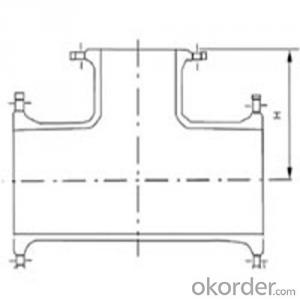
Ductile Iron Pipe Fittings All Socket Tee ISO2531/EN545 Made In China DN1800
- Loading Port:
- China main port
- Payment Terms:
- TT or LC
- Min Order Qty:
- 1 m.t.
- Supply Capability:
- 50000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
1.Ductile Iron Pipe Fittings Description :
Our product is an ideal material for connection, which is widely applied to construction machinery, chemical industry and other areas, especially in pipe linking system. It can be widely used under all conditions for a long time.
1) Pipe fittings confirm to ISO2531,K9 class,T type joint,6m long,with inside cements lining conform to ISO4179, outside Zinc spraying(130g/m2) and bitumen coating(70μm) conform to ISO8179.
2) Pipe fittings ends: Spigot and socket ends, with 100% SBR rubber gaskets accoding to ISO4633
2.Main Features of the Ductile Iron Pipe Fittings:
• Best Raw material
•Good service
•Competitive price
•High tensile Strength
•High corrosion resistance
•Pressure Resistence
•Anti-corrosion
•Installation is convenient
•Satisfy the highest hygienic standards
3.Ductile Iron Pipe Fittings Images:


4.Ductile Iron Pipe Fittings Specification:
Specification& Payment terms
Internal lining: ductile iron pipe fittings shall have an internal cement mortar lining in acc with ISO4179.
External coating: ductile iron pipe fittings shall be externally coated with metallic zinc spray plus a further layer of resin painting to ISO8179.
Gasket: 100% SBR/NBR/EPDM rubber gasket in accordance with ISO4633.
Packing: ductile iron pipes from DN100 to DN300 be bundled with steel belts, others are in bulk.
Payment term: L/C, T/T.
Packing: In bulk vessel or in container
5.FAQ:
We have organized several common questions for our clients,may help you sincerely:
1.Q: Why would you choose ductile iron pipe fittings rather than other pipe fittings materials?
A:The reasons are obvious for that not only ductile iron pipe fittings possesses the inherent strength and flexibility of ductile iron, combined with proven corrosion protection systems, but also the cost savings can be achieved from design to installation and commissioning.
2.Q:Why can you guarantee the inner of pipes can’t be corroded?
A: High alumina cement mortar lining and sulphate-resistant cement mortar lining. These two special linings are applicable to inner anti-corrosion for sewage pipes fittings, improving resistance to erosion of the sewage components.
- Q:Hi,Since ferritin stores iron as Fe 2+ (which is toxic to cells), how does it release the iron without causing cell death? Thank you!
- power surge from charging system could have blown out your brake light
- Q:I‘m a teenage girl and I‘m almost positive I have an iron deficiency, or anemia, or whatever.I get tired all the time, I‘m unusually pale, I have trouble walking a flight of stairs (I‘m not obese, my weight is normal for my height), and I can sleep for 10 hours but wake up as tired as I was before.How many milligrams of iron should I take? I heard if you take too many it can lead to death. and should I consult a doctor before doing so?
- if you are going to take tablets, then of coarse you need to consult your doctor. but before you do this you might think about how you are eating. not eating enough or extreme dieting can cause this (that's what happened to me). if i were you i would try eating more red meat before you decide you are going to take the tablets. this is a safer more natural way of getting the iron you need (as long as you don't eat to much of it). red meat is loaded with iron and girls need more iron than men because they menstruate (same goes for calcium). you lose a lot of iron and calcium when you bleed. Hope this helps! Please pick me for best answer!
- Q:what is a good flat iron at fair price and a good flat iron shine spray
- Chi (the hair product shine spray stuff comes with it) but its pretty expensive $140.00 but WELL worth it. Lifetime guarentee too.
- Q:Who would win and why?I say Iron Fist!
- Iron Fist would win. Nothing beats the power of the Chi.
- Q:Normally I have never enjoyed chewing or eating ice but as of the past week or two all I can think about is chewing and eating ice. About a month ago I did give blood. But other then that I have no other idea of why I might be iron deficient because normally I have high levels of iron. And doesn‘t chewing ice suddenly mean I have become iron deficient? Should I see a doctor if this is the case? I have no other symptoms that anything is wrong.
- Iron anemia can lead to dizziness and fatigue. There are good amounts of iron in oysters and mussels. Nuts are a good source of iron and also iron fortified cereals such as Weetbix. Wheat germ and wheat bran are both rich in iron. Perhaps you need an iron supplement.
- Q:I wanna do an experiment, and I wanna know if it‘s possible to grow iron sulfate crystals as it is for copper sulfate crystals. are ferrous sulfate and iron sulfate the same product??
- Hi! Iron sulfate is not correct. iron has 2 valences (II) AND (III). It can either be iron (II) sulfate or iron (III) sulfate. Ferrous sulfate is the IUPAC name for Iron (II) Sulfate. Ferrous Sulfate and Iron (II) Sulfate are the same (Product). Hope it helps.
- Q:What is iron III, and how does it combine with polyatomic ions? For example, what would be the chemical formula for the combination of iron III and C2H3O2- (acetate)?
- B - iron is not a group A metal, but rather a transition metal. Oxygen is a nonmetal anion.
- Q:I‘m rather stuck on this question:What mass of iron(II) sulfate would be needed to provide 28 grams of iron?I know that the mass of one mole of iron(II) sulfate is 152g, but I‘m not entirely certain of the question.All posts are appreciated!
- Iron (II) Sulfate formula is FeSO4 That is 1--Iron,1-Sulfur,4-Oxygen Atomic weights are Fe-56 S-32 O-16 (4 O- 64) Add them up and you get 152 thats why one mole weighs 152 grams So one mole of Iron(II) Sulfate has 56 grams of Iron in it. To get 28 grams of Iron would be one half mole or 76 grams of Iron (II) Sulfate. Make Sense ?
- Q:What is the colour of iron ions?Iron(II) ion greenIron(III) ion yellowRust (Iron oxide + water) black or brownExplain why the rust of iron is not green or yellow, but black or brown.
- Actually, the iron(III) ion, [Fe(H2O)6]3+, is an extremely pale purplish colour, as you will see if you grow a crystal of iron ammonium alum, NH4Fe(SO4)2.12H2O.This is because the ion is high spin d5, meaning that d-d transitions are spin-forbidden, and therefore even weaker than usual. The yellow colour of iron(III) in solution is due to species such as [Fe(III)(H2O)5OH]2+, which absorb strongly in the blue-near UV through charge transfer transitions, in which an electron is transferred from a coordinated anion to the reducible central ion. Compare the deep purple colour of permanganate, which has a highly irreducible central ion, but no d electrons at all. The pale green colour of Fe(II) is due to d-d transitions. The reason why the d orbitals are not degenerate in transition metal complexes is that they interact to different extents with the coordinated groups. Remember that when we talk about Fe2+ in aqueous solution, this is really just a lazy way of referring to the [Fe(H2O)6]2+ complex ion. The strong colours of iron oxides are due to charge transfer, including in some cases, such as magnetite, charge transfer between iron(II) and iron(III) in slightly different environments. Intervalence charge transfer of this kind is also responsible for the extremely deep colour of Prussian Blue.
- Q:Iron pills or b complex vit? Someone said they were simular it regaining iron. how?
- If you're a female, take folic acid also (it's a vitamin). That's what my doc put me on when I was way anemic.
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
Ductile Iron Pipe Fittings All Socket Tee ISO2531/EN545 Made In China DN1800
- Loading Port:
- China main port
- Payment Terms:
- TT or LC
- Min Order Qty:
- 1 m.t.
- Supply Capability:
- 50000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products
Hot Searches
Related keywords