Amino Trimethylene Phosphonic Acid Manufacturer Under ISO Test
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Product Description:
Amino tris(methylene phosphonic acid) / Amino Trimethylene Phosphonic Acid/ ATMP / 6419-19-8 / C3H12NO9P3
CAS No. 6419-19-8
Molecular Formula: N(CH2PO3H2)3
Molecular weight: 299.05
Structural Formula: 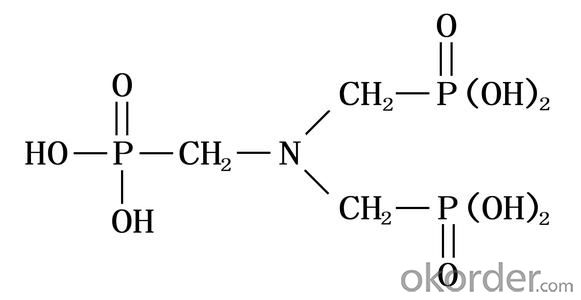
Properties:
ATMP has excellent chelation, low threshold inhibition and lattice distortion ability. It can prevent scale formation, calcium carbonate in particular, in water system. ATMP has good chemical stability and is hard to be hydrolyzed in water system. At high concentration, it has good corrosion inhibition.
ATMP is used in industrial circulating cool water system and oilfield water pipeline in fields of thermal power plant and oil refinery plant. ATMP can decrease scale formation and inhibit corrosion of metal equipment and pipeline. ATMP can be used as chelating agent in woven and dyeing industries and as metal surface treatment agent.
The solid state of ATMP is crystal powder, soluble in water, easily deliquescence, suitable for usage in winter and freezing districts. Because of its high purity, it can be used in woven & dyeing industries and as metal surface treatment agent.
Specification:
| Items | Index | |
|---|---|---|
| Standard | Solid | |
| Appearance | Clear, Colorless to pale yellow aqueous solution | White crystal powder |
| Active acid % | 50.0-51.0 | 95.0min |
| Chloride (as Cl-)% | 1.0 max | 1.0 max |
| pH value (1% solution) | 2.0 max | 2.0 max |
| Fe,mg/L | 10.0max | 20.0max |
| Density (20°C)g/cm3 | 1.31-1.35 | - |
| Colour APHA (Hazen) | 30.0max | - |
Application range&using method:
ATMP is usually used together with other organophosphoric acid, polycarboxylic acid and salt to built all organic alkaline water treatment agent. ATMP can be used in many different circulating cool water system. The recommended dosage is 5-20mg/L. As corrosion inhibitor, The recommended dosage is 20-80mg/L.
Package and Storage:
ATMP liquid: Normally In 30kg or 250kg net Plastic Drum;ATMP solid: 25kg inner liner polyethylene (PE) bag, outer plastic woven bag, or confirmed by clients request.Storage for ten months in room shady and dry place.
Safety Protection:
ATMP is Acidity, Avoid contact with eye and skin, once contacted, flush with water.
Shipping Date: Within 7-10 workdays after receiving your deposit.
Our Service:
Own Lab and joint venture factory.
Superb r&d team;Safety standardization production.
Rich experience in export and strong logistical support.
Good relationship with many large domestic pharmaceutical factory.
Perfect service, perfect supply chain.
- Q:What is a catalyst in a chemical reaction?
- A catalyst is a compound in chemistry (it can be an acid or temperature or a base or a metal or anything, pressure anything) that shifts the reaction towards one product or the other... In simple words. If you want to obtain something, e.g. water, then you can obtain it in different timings, ie in 2000 years, but if you want to obtain it in 2 hours instead of 2000 years then you add a catalyst, e.g. you heat the reaction to speed it up, you add an acid, or a base etc. Some catalysts also act on the regioselectivity of a compound thru preferring the formation of a stereo-isomer to another. E.g. if you want to obtain S-Thalidomide instead of R-Thalidomide you use a particular catalyst etc...
- Q:how do catalysts help in green chemistry?
- Catalysts allow more efficient conversion of products in irreversible reactions, or they allow for the faster attainment of equilibrium in equilibrium reactions, thereby reducing time, raw material waste and emissions. Also, because catalysts are reusable, they can be recycled.
- Q:i keep messing up on those 2 simple things haha i would apprecaite some help.
- enzymes help biochemical reactions proceed at a faster rate than normal in a physiological system, catalysts or sometimes referred to as subunits, metals and other ligands, bind enzymes, and can have a positive and negative effect on the rate of a reaction. search them on wikipedia!
- Q:Chemical catalyst system baa?
- Can speed up or slow down the reaction rate without participating in the reaction of the material
- Q:give an example of how a catalysts speeds up the rate of reaction?? thank you!!?
- Catalysts speed up a reaction, but at the end of it, it's chemically unchanged. You usually have the same mass at the end of the reaction. For example.. Consider the decomposition of Hydrogen Peroxide: 2H2O2 -2H2O + O2 I hope you got how to represent it =D So.. This reaction is very slow. You can try it out in the lab. So when we add a catalyst, the reaction speeds up. The catalyst used here is MnO2.. Manganese dioxide. Well.. The Enzymes in our body are also Catalysts. They speed up the Biological Reactions taking place inside out body. I hope this answers your question. :) Cheers
- Q:Will the catalyst decompose during the reaction between two substances? Exp:the decomposition of hydrogen peroxide.Will the manganese 4 oxide decompose?
- Catalysts are not used/destroyed in any reactions, it merely speeds up the process by lowering the reaction activation energy. It functions by being able to weaken or break the required bonds necessary in the chemical reaction (thus lowering activation energy) through temporary and weak bonding to form a complex. In this case the H2O2 molecule will bind with the MnO2 molecule due to the complimentary sites (thus forming a complex) to weaken the bonds for decomposition, but after decomposition the products (oxygen and water molecules) break off from the catalyst (as there are no more complementary sites with them) thus the catalyst will not be destroyed.
- Q:Especially how can i explain the experiment with a paper and 2 paperclips with the paper acting as the catalyst.
- A catalyst acts on one material to activate it towards reaction with another material that it would not otherwise spontaneously react with (it lowers the reaction's activation barrier). For instance, the 2 paperclips may not want to react with each other, but if the paper attaches to one, it becomes more reactive and it will now clip onto the other paperclip. The catalyst then leaves (paper is detached) which is called catalyst regeneration, which goes on to activate another molecule in the same fashion. A common example is using Lewis acid catalysts to activate carbonyls by coordinating to the oxygen so that the the carbon becomes more electrophilic for attack by some nucleophile.
- Q:Are biological enzymes harmful to humans?
- Biological enzymes through scientists more than a century of research, usually known as more than 3,000 kinds of enzymes, the current application of biological enzymes in the textile a wide range of technology, fiber modification, silk degumming, raw hemp (ramie, linen, Kenaf) degumming, dyeing and finishing of the desizing, refining, finishing and net cleaning processing, textile printing and dyeing wastewater treatment and garment processing and other aspects of the application. Enzyme technology has a unique advantage in improving dyeing and finishing processes, saving energy, reducing environmental pollution, improving product quality, adding value and developing new raw materials. At present in the textile processing using a wide range of enzyme preparations are mainly cellulase, protease, amylase, pectinase, lipase, peroxidase, laccase, glucose oxidase eight categories.
- Q:Cl + O3 ---> ClO + O2O + ClO ---> Cl + O2= O + O3 ----> 2O2What is the catalyst? The intermediate?How do you know which is which? If the rate law is rate=k [O3] [Cl]determine:a) the overall order.b) unit for k.c) the rate determining step, justify your answer.
- Cl is the catalyst. ClO the intermediate. The catalyst is the component which does not change in overall reaction. He forms some intermediate component(s) with the reactants. In the later reaction steps the intermediate(s) react forming the catalyst in its original state. (a) The overall order is the sum of the orders with respect to the components: n = 1 +1 = 2 (b) the unit of the rate of reaction is r [=] mol/ (Ls) (more general mol per unit time and volume) compare dimensions mol / (Ls) [=] k · mo/L · mol/L =k [=] L/(s mol) (more general unit volume per unit time and mole) (c) First reaction For elementary reaction steps the order of the reaction rate with respect to a reactant is equal to stoichiometric coefficient. Hence the rate of first reaction is: r? = k?·[Cl]·[O?] Overall rate is given by the rate determining step, while other reaction steps are in equilibrium: r = r? = k?·[Cl]·[O?] If second reaction is the rate determine step r? = k?·[O]·[ClO] while reaction 1 is at equilibrium K? = ( [ClO]·[O?] ) / ( [Cl]·[O?] ) =[ClO] = K?·( [Cl]·[O?] ) / [O?] the overall rate would be: r = r? = k?·[O]·[ClO] = K?·k?·[O]·[Cl]·[O?] / [O?] = k·[O]·[Cl]·[O?] / [O?] That doesn't match the observed rate law
- Q:What happens to a catalyst after a chemical reaction?
- If it is only a catalyst, then by definition it will still be there at the end.
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
Amino Trimethylene Phosphonic Acid Manufacturer Under ISO Test
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products
Hot Searches
Related keywords
























